Description #
An online version of the SOGC Guidelines to the diagnosis, evaluation and management of the hypertensive disorders of pregnancy.
Learning Objectives #
At the end of this session the care provider will be able to:
- 1. Define the diagnosis and classification of hypertension disorders in pregnancy (HDP).
- 2. Define the predictors and identify prevention stategies for HDP.
- 3. Define maternal and fetal prognaosis in preeclampsia.
- 4. Identify appropriate antenatal treatment for HDP.
- 5. Identify appropriate postpartum treatment of longer term follow-up.
The hypertensive disorders of pregnancy (HDP) are a leading cause of maternal and perinatal mortality and morbidity, in Canada1 and internationally2;3. In 1994, the Canadian Hypertension Society (CHS) initiated a consensus project on the diagnosis, evaluation and management of the hypertensive disorders of pregnancy. The resulting guidelines, published in the CMAJ in 1997 and endorsed by the Society of Obstetricians and Gynaecologists of Canada (SOGC), were instrumental in changing the classification of the hypertensive disorders of pregnancy, adding ‘adverse conditions’ of maternal and perinatal morbidity. The guidelines have been widely cited, and they informed the updates of the American4 and Australasian5 guidelines, both published in 2000. In 2005, the SOGC, with representation from the CHS (AL) and from the BC Reproductive Care Programme (BCRCP), initiated a process to update the Canadian guidelines.
The new guidelines are more comprehensive and target a wider audience than previously.
- The document is self-contained, with three major parts entitled: ‘Diagnosis, Evaluation, and Classification’; ‘Prevention, Prediction (new), and Prognosis (new)’; and ‘Treatment’ (which combines the previous ‘Non-pharmacological and Pharmacological Therapy’ sections).
- The format has been changed to one of recommendations and comments, consistent with current SOGC and Canadian Hypertension Education Programme (CHEP) guidelines (Appendix 1).
- The classification of the HDP has been simplified to that of pre-existing or gestational hypertension, with subcategories of ‘with co-morbidities’ (to better inform antihypertensive therapy of non-severe hypertension) or ‘with pre-eclampsia’ (to achieve brevity).
- The management section balances the risks and benefits of interventions; it also includes information about anaesthesiology, platelet transfusion and detailed immediate and long-term postpartum follow-up.
- Judicious fluid administration in pre-eclampsia is a consistent theme, given that pulmonary edema is a potentially avoidable, yet leading, cause of maternal morbidity in pre-eclampsia6, just as severe hypertension is to stroke.
- Areas of ongoing investigation are highlighted.
As per the usual practice of the SOGC, the system used for grading the quality of evidence was that of the Canadian Task Force on the Periodic Health Exam (Appendix 1). In evaluating the evidence, consideration was given to other international guidelines7;8. The new guidelines reflect the leadership role of Canadian investigators in the area of HDP. During the preparation of this document, Canadians have served as President of the International Society for the Study of Hypertension in Pregnancy (ISSHP, J-M M), on the Executive Council of the ISSHP (J-M M, LAM, PvD), and as Vice-President of the North American Society for the Study of Hypertension in Pregnancy (NASSHP, PvD).
In summary, the purpose of these guidelines is to summarize the quality of the evidence to date and provide a reasonable approach to the diagnosis, evaluation and treatment of HDP. There are still many areas where evidence is insufficient to guide clinical practice. These deficiencies need to be addressed in future research studies.
Methods #
Canadian obstetricians and internists knowledgeable about HDP and guideline development participated in the project. Invitations to participate took into account geographical representation, previous involvement in developing HDP guidelines, ongoing interest and expertise in the HDP, and membership in CHS and/or SOGC. Four subgroups were struck, each with a leader and approximately five participants. Through consensus, the work of each subgroup was divided amongst its members, each of whom took primary responsibility for some aspect(s) of the work.
The literature reviewed included the original HDP guidelines9-11 and their reference lists, and an update from 1995. Each subgroup leader provided the CHS (eadya@mcmaster.ca) with key words for a subgroup literature search of MEDLINE (1995-2005). Searches were subsequently updated by subgroup members in 2006. Articles were restricted to those published in French or English. The keywords used are listed in Appendix 2. The concepts explored for ‘pregnancy’ and ‘hypertension’ were: diagnosis, evaluation, classification, prediction (using clinical and laboratory markers), prevention, prognosis, treatment of hypertension, other treatments of the hypertensive disorders, general management issues (such as mode of delivery and anaesthetic considerations), and postpartum follow-up (for subsequent pregnancy(ies)) and long-term. The relevant list of potential articles was provided to subgroup participants, each of whom took responsibility for drafting the recommendations and comments for a specific aspect(s) of his/her subgroup’s work.
A focus was placed on consideration of randomized controlled trials (RCTs) for therapy and evaluation of substantive clinical outcomes (rather than surrogate markers such as laboratory values). Recommendations were graded according to algorithms used by the Canadian Task Force on the Periodic Health Examination (Appendix 1)12.
Progress was reported directly to the chair (LAM) who put together the final document and incorporated revisions. Subgroup work was reviewed first by teleconferences, followed by a face-to-face meeting of all subgroups SOGC (June 2006, Vancouver, BC). Subsequently, each participant’s assigned work (of recommendations and comments) was sent to the chair (LAM) for amalgamation into a single working document. This was circulated for feedback by e-mail (November 2006). Comments were received by e-mail and discussion of more contentious issues occurred by teleconference (two, December 2006). A face-to-face meeting was held in Winnipeg (March 2007) after which a final draft document was produced. By teleconferences of committee members, the final grading of the recommendations was performed using methodological criteria from theCanadian Task Force on the Periodic Health Examination (Appendix 1). The resulting document was sent to the Guidelines and Perinatal Committees of SOGC for review (May 2007), the British Columbia Reproductive Care Programme (BCRCP) (May 2007), and the Obstetric section of the Canadian Anesthesiologists Society (June 2006 and 2007). After revisions were received (# 2007) and considered by e-mail exchange and teleconference with all committee members (including the CHS representative), a final document was produced for publication (# 2007).
Diagnosis and Classification #
The classification of the hypertensive disorders of pregnancy is based on the two most common manifestations of pre-eclampsia: hypertension and proteinuria. Accordingly, the measurement of blood pressure and proteinuria, and the diagnosis of hypertension and clinically significant proteinuria, are described in detail.
Measurement of BP #
Recommendations:
- BP should be measured in the sitting position with the arm at the level of the heart (II-2, A).
- An appropriately sized cuff (i.e., length of 1.5 times the circumference of the arm) should be used (II-2, A).
- Korotkoff phase V should be used to designate diastolic BP (I, A).
- If BP is consistently higher in one arm, the arm with the higher values should be used for all BP measurements (III, B).
- BP can be measured using a mercury sphygmomanometer , calibrated aneroid device, or an automated BP device that has been validated for use in pre-eclampsia (II-2, A).
- Automated BP machines may underestimate BP in pre-eclampsia and comparison with mercury sphygmomanometry or aneroid device is recommended for an individual woman (II-2, A).
- Automated BP monitoring (by 24-hour or home measurement) may be useful to detect isolated office (‘white coat’) hypertension’ (II-2, B).
- Patients should be instructed on proper BP measurement technique if they are to perform home BP monitoring (III, B).
Comments:
We have focused in measurement issues that are specific to pregnancy. The reader should refer to the most recent CHEP document for general guidelines13.
Measurement should follow standardised technique, as outside pregnancy14. It is preferable to have women rest for five minutes. In particular, Korotkoff phase V should be used for designation of diastolic BP as it is more reliable15, and with its use (compared with use of phase IV), pregnancy outcome is similar16. This recommendation replaces the previous to use both phase IV and phase V. Phase IV (muffling) should be used for diastolic BP only if Korotkoff sounds are audible as the level approaches 0 mmHg. A cuff that is too small (e.g., such that the white lines do not cross) will overestimate sBP by 7-13mmHg and dBP by 5-10mmHg. A cuff should never be placed over clothing. Women should be in the sitting position which gives the highest BP; supine positioning has the potential to cause hypotension, and left lateral positioning has the potential to give the lowest BP value because the right arm is frequently elevated above the level of the heart during BP measurement17. Any arm-arm differences should be documented and if the BP is consistently higher in one arm, that arm should be used for all BP measurements18.
BP can be measured using a mercury sphygmomanometer, aneroid device, or automated (usually oscillometric) BP device, as mercury sphygmomanometers have been eliminated from many institutions. When choosing a BP measurement device, considerations include: observer error, validation, disease specificity, and recalibration on a regular basis (i.e., every six months).
Recalibration involves comparing readings taken with a given device with a mercury manometer. Aneroid devices must be recalibrated every two years against mercury devices. This is performed by the biomedical department of hospitals but must be arranged separately by those practitioners with private offices.
Validation is undertaken to determine the accuracy of a device, at all levels of BP readings, on several occasions, and for women with different HDPs19. Validation must be done particularly in women with pre-eclampsia for two reasons. First, the detection of pre-eclampsia is the major purpose of BP measurement in pregnancy. Second, women with pre-existing hypertension have an approximately 20% risk of pre-eclampsia20-24, and women with gestational hypertension may evolve into a typical pre-eclampsia25-30. Automated BP measurement devices will eliminate observer error. However, only some devices have been validated in pregnancy31 and in pre-eclampsia, specifically32. Automated devices may underestimate BP in pre-eclampsia by an average of 5mmHg in systolic and diastolic, but there is wide variation33.
Most errors in office BP measurements are operator dependent and correctable34. However, ambulatory measurements have gained popularity. 24-hour ambulatory BP monitoring (ABPM) or serial BP measurements in an obstetrical day unit may identify women who have isolated office hypertension. Compared with persistently hypertensive women, women with isolate office hypertension are at lower risk of maternal and perinatal complications35-39. However, 24-hr ABPM is of only modest use for an individual woman, because of negative predictive values that only modestly decrease the risk of adverse outcomes such as severe hypertension, preterm delivery, and admission to NICU40-42. Home BP monitoring is widely available, economical, comfortable, easy to repeat when disease evolution is suspected, and pregnant women prefer it to 24-hr ABPM43. However, values have not been validated against adverse pregnancy outcomes.
Therefore, at present, there is insufficient information to define the role of either method of ambulatory BP monitoring in hypertensive (or normotensive) pregnancy. To date, no RCT has been performed to assess the impact of any type of ambulatory BP measurement on maternal or perinatal outcomes44.
Diagnosis of Hypertension #
Recommendations:
- The diagnosis of hypertension should be based on office or in-hospital BP measurements (II-2, B).
- Hypertension in pregnancy should be defined as a diastolic BP of ≥90mmHg, based on the average of at least two measurements, taken in the same arm (II-2, B).
- Women with a systolic BP of ≥140mmHg should be followed more closely for development of diastolic hypertension (II-2, B).
- Severe hypertension should be defined as a systolic BP of ≥160mmHg or a diastolic BP of ≥110mmHg (II-2, B).
- For non-severe hypertension, serial BP measurements should be performed before a diagnosis of hypertension is made (II-2, B).
- For severe hypertension, repeat measurement should be confirmed in 15 minutes (III, B).
- ‘White coat’ (isolated office) hypertension should be defined as office dBP of ≥90mmHg, but home BP <135/85mmHg (III, B).
Comments:
The definition of hypertension in pregnancy is dBP ≥90mmHg, by office measurement. A dBP of 90mmHg identifies a level above which perinatal morbidity is increased in non-proteinuric hypertension, and dBP is a better predictor of adverse pregnancy outcomes than is sBP45;46. Non-severely elevated BP should be confirmed by repeat measurement, preferably on more than one visit, as 30-70% of women with an office BP of ≥140/90mmHg have subsequently normal BP on subsequent measurements on the same visit, after serial measurement in an obstetrical day unit, after home BP monitoring47-50. Whether the BP is repeated over hours, days, or weeks will depend on the underlying HDP.
Systolic BP (sBP) was previously excluded from the definition of hypertension in pregnancy for several reasons. First, it is subject to more variation than is dBP. Second, it is usually increased along with dBP51. Third, there is the potential for overlabelling and seeing women more frequently than necessary. However, even an intermittently elevated sBP is a risk marker for later development of gestational hypertension52, so elevated sBP should trigger closer follow-up and investigation as appropriate.
Defining severe hypertension as a systolic BP ≥160mmHg (instead of ≥170mmHg) is based on the fact that sBP ≥160mmHg is associated with an increased risk of stroke in pregnancy53;54.
A relative rise in BP is not part of the definition of hypertension, given that it is within the variation in BP seen in all trimesters of pregnancy, and there is a high false positive rate for suspected pre-eclampsia55. Mean arterial pressure (MAP) is not part of the definition of hypertension in pregnancy as it is cumbersome to calculate.
If home BP monitoring is used to identify women with isolated office hypertension, then ideally, normal home BP values should be confirmed by 24hr ambulatory BP monitoring. As criteria for normality have varied, use of the widely accepted threshold (outside of pregnancy) of <135/85mmHg for normal home BP measurements is recommended56 (see discussion in ‘BP measurement’).
Measurement of Proteinuria #
Recommendations:
- All pregnant women should be assessed for proteinuria (II-2, B).
- Urinary dipstick testing may be used for screening for proteinuria when the suspicion of pre-eclampsia is low (II-2, B).
- Testing for proteinuria by urinary protein:creatinine ratio or 24hr urine collection is encouraged when there is a suspicion of pre-eclampsia, including hypertensive pregnant women with rising BP or normotensive pregnant women with symptoms or signs suggestive of pre-eclampsia (II-2, A).
Comments:
Most testing for urinary protein is performed to screen for pre-eclampsia in hypertensive women or those at increased risk of pre-eclampsia, although urinary protein screening is used in early pregnancy to detect pre-existing renal disease. The current recommendations have been revised to reflect the critical fact that proteinuria is but one diagnostic criterion for pre-eclampsia. The end-organ complications of pre-eclampsia may occur in the absence of proteinuria; for example, 20% of women who develop eclampsia will have had only hypertension in the week preceding their seizure; 10% will have had neither, and 10% only proteinuria57. There is also the need for both efficiency and economy in clinical care.
There are many options for diagnosis of proteinuria, including urinary dipstick testing, urinary protein:creatinine ratio, and various timed urine collections (most commonly, 24hr). We do not know the method that best identifies women at increased risk of maternal and/or perinatal complications. However, in a retrospective study, increasing number of pluses of urinary dipstick proteinuria was associated with increasing risk of adverse maternal outcomes58. Most research has focussed on methods that best match the quantification of urinary protein by 24hr urine collection, considered to be the gold standard. However, 24hr urine collection is time-consuming, inconvenient, and often not complete (as assessed by collection of 12-18% of the ideal body weight as urinary creatinine)59. For diagnosis of proteinuria, these logistical considerations have prompted the National Kidney Foundation to abandon timed collections in favour of the spot urine samples.
Diagnosis of clinicially significant Proteinuria #
Recommendations:
- Urinary dipstick proteinuria of ≥2+ should make the clinician suspicious of proteinuria (II-2, A).
- Proteinuria should be defined as ≥0.3g/d in a 24-hour urine collection or ≥30mg/mmol urinary creatinine in a spot (random) urine sample (II-2, B).
Comments:
The upper limit of normal 24hr urine protein excretion is 0.3g/d and is based on a 95% CI for urinary protein in pregnancy. It is used by convention; however, a urinary protein measurement of ≥0.5g/d is actually a better predictor of adverse clinical outcome60.
The urinary protein:creatinine ratio (U PCR) has been accepted for diagnosis by the International (ISSHP) and Australasian (ASSHP) pregnancy hypertension societies. Ideally, it should be performed in the morning but not on the first voided urine; however, timing may not be critical in pregnancy61. The reported cut-off varies from 19 to 55 mg/mmol in ten studies (1,004 hypertensive women)62-71. For a cut-off of 30mg/mmol urinary creatinine (as recommended by the ASSHP), and among women with a HDP specifically, the sensitivities and specificities were 0.84 [95% CI of 0.78, 0.90] and 0.74 [0.71, 0.78], respectively72. Efforts are underway to improve the standardization of urinary protein and serum creatinine measurement across laboratories73.
Urinary dipstick testing is inexpensive, easy and widely used. Its usefulness is uncertain for screening either women with hypertension, or those who are at increased risk of pre-eclampsia. A negative or trace value should not be ignored in a woman with new hypertension or symptoms or signs suggestive of pre-eclampsia; 12% of ‘negative/trace’ results will be a false negative as assessed against 24-hr proteinuria of 0.3g/d74, and these women may have pre-eclampsia without proteinuria anyway.
For the detection of significant proteinuria, urinary albumin:creatinine ratio (U ACR) generally performed well (in comparison with 24hr urinary protein excretion) in three prospective studies75-77 but not in a fourth78 (321 hypertensive women). More information is needed before clinical use of the urinary ACR can be recommended.
It is not clear that there is a role for the quantification of proteinuria in pregnancy, for purposes of prognostication which is discussed under ‘Prognosis of Pre-eclampsia’. If quantification is sought, then 24hr urine collection should be used as the U PCR is less reliable at high levels of proteinuria.
Classification of the HDP #
Recommendations:
- The hypertensive disorders of pregnancy should be classified as pre-existing or gestational hypertension, based on different diagnostic and therapeutic issues (Table 1) (II-2, B).
- The presence/absence of pre-eclampsia must be ascertained given its clear association with more adverse maternal and perinatal outcomes (Table 1) (II-2, B).
- In women with pre-existing hypertension , ‘pre-eclampsia’ should be defined as resistant hypertension, new/worsening proteinuria, or one or more of the other ‘adverse conditions’ (Table 1) (II-2, B).
- In women with gestational hypertension, ‘pre-eclampsia ’ should be defined as new-onset proteinuria or one or more of the other ‘adverse conditions’ (Table 1) (II-2, B).
- ‘Severe pre-eclampsia’ should be defined as pre-eclampsia: with onset before 34 weeks’ gestation, with heavy proteinuria, or with one or more ‘adverse conditions’ (II-2, B).
- The term ‘PIH’ (pregnancy-induced hypertension) should be abandoned, as its meaning in clinical practice is unclear (III, D).
Comments:
The purpose of classification is to facilitate communication among care-givers, and to create meaningful groups with different prognoses, considerations for surveillance, and/or outcomes. As such, the classification system for the hypertensive disorders of pregnancy has been simplified.
Using population-based data, approximately 1% of pregnancies are complicated by pre-existing hypertension, 5-6% by gestational hypertension without proteinuria, and 1-2% by pre-eclampsia79. It can be expected that these numbers will increase given the trend towards an older and more obese obstetric population.
Hypertension is classified as pre-existing or gestational (Table 1). Pre-existing hypertension pre-dates pregnancy or appears before 20 weeks, and gestational hypertension appears at or after 20 weeks. There are two subgroups: ‘with co-morbid conditions’ and ‘pre-eclampsia’. Edema and weight gain remain excluded from the definition of pre-eclampsia. Edema, even facial, is neither sensitive nor specific for pre-eclampsia80;81. Neither edema nor weight gain are significantly associated with perinatal mortality and morbidity45;80. This liberal definition of pre-eclampsia is meant to signal a need for heightened maternal and fetal surveillance, recognizing that all of the ‘adverse conditions’ do not carry equal weight (e.g., eclampsia and persistent, new/unusual headache).
‘Severe pre-eclampsia’ corresponds to pre-eclampsia: with onset before 34 weeks’ gestation, with heavy proteinuria (3-5g/d according to other international guidelines), or with one or more ‘adverse conditions’. This definition is consistent with American guidelines82 and those from the ISSHP80, with the exception of the gestational age criterion (see ‘Prognosis of pre-eclampsia’ and ‘Place of Care’, and specific therapy). Although the magnitude of proteinuria has not been consistently associated with worse maternal or perinatal prognosis, proteinuria is retained in the definition of ‘severe pre-eclampsia’ for face validity, until there are definitive data to indicate that heavy proteinuria should be removed.
Women with pre-existing hypertension have a 10-20% risk of developing pre-eclampsia, defined by resistant hypertension, new/worsening proteinuria, or one or more ‘adverse condition’ (Table 1)83-87. Women with certain co-morbidities (e.g., renal disease or pre-existing diabetes mellitus) are also at increased risk88. Women with gestational hypertension with onset before 34 weeks (as opposed to onset at ≥34 weeks) are more likely to evolve into a pre-eclampsia picture, with rates of about 35%26;27;29;89-91.
Table on page S8 needs to be inserted. Last one illegible.
With ‘co-morbid conditions’
‘With co-morbid conditions’ refers to conditions that are strong indications for more aggressive antihypertensive therapy outside of pregnancy92, and as such, they warrant special BP treatment thresholds and goals in pregnancy. ‘Co-morbid conditions’ are highlighted because they constitute indications for antihypertensive therapy over the short-term, outside pregnancy. These are usually major cardiovascular risk factors, such as type I or II (but not gestational) diabetes, renal parenchymal or vascular disease, or cerebrovascular disease.
With pre-eclampsia
The term, ‘pre-eclampsia’ has been re-introduced for its brevity and because of its international use. It corresponds to the previous terms of:
- pre-existing hypertension with superimposed gestational hypertension, proteinuria and/or an ‘adverse condition’(s),
- gestational hypertension with proteinuria, or
- gestational hypertension (without proteinuria) with one/more ‘adverse condition’(s).
The changes have been made for clarity. First, the term ‘superimposed’ is not used, but the criteria for the diagnosis of pre-eclampsia in women with pre-existing hypertension have been clarified. ‘Resistant’ hypertension refers to that which requires three antihypertensive medications, after 20 weeks’ gestation. Second, the classification emphasizes that there is significant clinical overlap, that women may meet criteria for more than one subgroup, and that evolution may occur over time. A final diagnosis of the type of HDP is retrospective, following the postpartum period.
All hypertension societies regard pre-eclampsia as a hypertensive disorder most commonly defined by new-onset proteinuria, and potentially, other end-organ dysfunction. A ‘restrictive’ definition defines pre-eclampsia as gestational hypertension with proteinuria, and this is often used by the research community and endorsed for this purpose by the International Society for the Study of Hypertension in Pregnancy (ISSHP)93. An ‘inclusive’ definition defines pre-eclampsia as gestational hypertension with proteinuria or typical end-organ dysfunction. Both these guidelines and the Australasians ones use this ‘inclusive’ definition94. Although the American guidelines use a ‘restrictive’ definition of pre-eclampsia, they also state that end-organ dysfunction makes the diagnosis of pre-eclampsia “highly suspect”95.
‘Adverse conditions’ reflect pre-eclampsia related direct fetal complications (e.g., oligohydramnios), direct maternal systemic end-organ complications (e.g., eclampsia), or conditions that significantly heighten the risk of maternal complications (e.g., serum albumin <20g/L) (Table 1).
The ‘adverse conditions’ have been modified. Elevated creatinine has been added. Both oliguria and proteinuria >3g/d have been removed. Oliguria is non-specific and has many causes, including high ADH levels after stress or surgery. Also, pulmonary edema from fluid administration is a major cause of death in women with pre-eclampsia96. Oliguria (<15mL/hr) should be tolerated, at least over the first 6 hours postpartum, in women who do not have pre-existing renal disease. Although there is a continuum of risk between greater proteinuria and more adverse outcomes80;97;98, there is no clear cut-off. (Use of urinary protein quantification for prognostication in pre-eclampsia is discussed under ‘Prognosis of Pre-eclampsia’.) A threshold for low serum albumin of <20g/L has been used as the point at which edema develops from hypoproteinemia alone99-101.
Hyperuricaemia has not been included as an ‘adverse condition’, but was considered because of its association with pregnancy complications that is at least as strong as proteinuria80;102. To date, serum uric acid has not predicted adverse maternal outcomes in pre-eclampsia103.
Gestational age has not been listed as an ‘adverse condition’. However, onset of hypertension at <34 weeks is a risk marker for evolution of gestational hypertension to pre-eclampsia, and is associated with an increased risk of maternal and perinatal complications26;27;29;104-106.
Pre-eclampsia is not just hypertension
Understanding the pathogenesis of pre-eclampsia is key to understanding the multi-system and varied clinical manifestations of pre-eclampsia. The most popular theory for the pathogenesis of pre-eclampsia describes a two-stage process, which ultimately results in a mismatch between uteroplacental supply and fetal demands, leading to maternal endothelial cell dysfunction and the maternal (and fetal) manifestations of pre-eclampsia (Figure 1)107. For details, see the reviews by Roberts JM et al 108;109.
Classification of the HDP #
Recommendations:
- The hypertensive disorders of pregnancy should be classified as pre-existing or gestational hypertension, based on different diagnostic and therapeutic issues (Table 1) (II-2, B).
- The presence/absence of pre-eclampsia must be ascertained given its clear association with more adverse maternal and perinatal outcomes (Table 1) (II-2, B).
- In women with pre-existing hypertension , ‘pre-eclampsia’ should be defined as resistant hypertension, new/worsening proteinuria, or one or more of the other ‘adverse conditions’ (Table 1) (II-2, B).
- In women with gestational hypertension, ‘pre-eclampsia ’ should be defined as new-onset proteinuria or one or more of the other ‘adverse conditions’ (Table 1) (II-2, B).
- ‘Severe pre-eclampsia’ should be defined as pre-eclampsia: with onset before 34 weeks’ gestation, with heavy proteinuria, or with one or more ‘adverse conditions’ (II-2, B).
- The term ‘PIH’ (pregnancy-induced hypertension) should be abandoned, as its meaning in clinical practice is unclear (III, D).
Comments:
The purpose of classification is to facilitate communication among care-givers, and to create meaningful groups with different prognoses, considerations for surveillance, and/or outcomes. As such, the classification system for the hypertensive disorders of pregnancy has been simplified.
Using population-based data, approximately 1% of pregnancies are complicated by pre-existing hypertension, 5-6% by gestational hypertension without proteinuria, and 1-2% by pre-eclampsia79. It can be expected that these numbers will increase given the trend towards an older and more obese obstetric population.
Hypertension is classified as pre-existing or gestational (Table 1). Pre-existing hypertension pre-dates pregnancy or appears before 20 weeks, and gestational hypertension appears at or after 20 weeks. There are two subgroups: ‘with co-morbid conditions’ and ‘pre-eclampsia’. Edema and weight gain remain excluded from the definition of pre-eclampsia. Edema, even facial, is neither sensitive nor specific for pre-eclampsia80;81. Neither edema nor weight gain are significantly associated with perinatal mortality and morbidity45;80. This liberal definition of pre-eclampsia is meant to signal a need for heightened maternal and fetal surveillance, recognizing that all of the ‘adverse conditions’ do not carry equal weight (e.g., eclampsia and persistent, new/unusual headache).
‘Severe pre-eclampsia’ corresponds to pre-eclampsia: with onset before 34 weeks’ gestation, with heavy proteinuria (3-5g/d according to other international guidelines), or with one or more ‘adverse conditions’. This definition is consistent with American guidelines82 and those from the ISSHP80, with the exception of the gestational age criterion (see ‘Prognosis of pre-eclampsia’ and ‘Place of Care’, and specific therapy). Although the magnitude of proteinuria has not been consistently associated with worse maternal or perinatal prognosis, proteinuria is retained in the definition of ‘severe pre-eclampsia’ for face validity, until there are definitive data to indicate that heavy proteinuria should be removed.
Women with pre-existing hypertension have a 10-20% risk of developing pre-eclampsia, defined by resistant hypertension, new/worsening proteinuria, or one or more ‘adverse condition’ (Table 1)83-87. Women with certain co-morbidities (e.g., renal disease or pre-existing diabetes mellitus) are also at increased risk88. Women with gestational hypertension with onset before 34 weeks (as opposed to onset at ≥34 weeks) are more likely to evolve into a pre-eclampsia picture, with rates of about 35%26;27;29;89-91.
Table on page S8 needs to be inserted. Last one illegible.
With ‘co-morbid conditions’
‘With co-morbid conditions’ refers to conditions that are strong indications for more aggressive antihypertensive therapy outside of pregnancy92, and as such, they warrant special BP treatment thresholds and goals in pregnancy. ‘Co-morbid conditions’ are highlighted because they constitute indications for antihypertensive therapy over the short-term, outside pregnancy. These are usually major cardiovascular risk factors, such as type I or II (but not gestational) diabetes, renal parenchymal or vascular disease, or cerebrovascular disease.
With pre-eclampsia
The term, ‘pre-eclampsia’ has been re-introduced for its brevity and because of its international use. It corresponds to the previous terms of:
- pre-existing hypertension with superimposed gestational hypertension, proteinuria and/or an ‘adverse condition’(s),
- gestational hypertension with proteinuria, or
- gestational hypertension (without proteinuria) with one/more ‘adverse condition’(s).
The changes have been made for clarity. First, the term ‘superimposed’ is not used, but the criteria for the diagnosis of pre-eclampsia in women with pre-existing hypertension have been clarified. ‘Resistant’ hypertension refers to that which requires three antihypertensive medications, after 20 weeks’ gestation. Second, the classification emphasizes that there is significant clinical overlap, that women may meet criteria for more than one subgroup, and that evolution may occur over time. A final diagnosis of the type of HDP is retrospective, following the postpartum period.
All hypertension societies regard pre-eclampsia as a hypertensive disorder most commonly defined by new-onset proteinuria, and potentially, other end-organ dysfunction. A ‘restrictive’ definition defines pre-eclampsia as gestational hypertension with proteinuria, and this is often used by the research community and endorsed for this purpose by the International Society for the Study of Hypertension in Pregnancy (ISSHP)93. An ‘inclusive’ definition defines pre-eclampsia as gestational hypertension with proteinuria or typical end-organ dysfunction. Both these guidelines and the Australasians ones use this ‘inclusive’ definition94. Although the American guidelines use a ‘restrictive’ definition of pre-eclampsia, they also state that end-organ dysfunction makes the diagnosis of pre-eclampsia “highly suspect”95.
‘Adverse conditions’ reflect pre-eclampsia related direct fetal complications (e.g., oligohydramnios), direct maternal systemic end-organ complications (e.g., eclampsia), or conditions that significantly heighten the risk of maternal complications (e.g., serum albumin <20g/L) (Table 1).
The ‘adverse conditions’ have been modified. Elevated creatinine has been added. Both oliguria and proteinuria >3g/d have been removed. Oliguria is non-specific and has many causes, including high ADH levels after stress or surgery. Also, pulmonary edema from fluid administration is a major cause of death in women with pre-eclampsia96. Oliguria (<15mL/hr) should be tolerated, at least over the first 6 hours postpartum, in women who do not have pre-existing renal disease. Although there is a continuum of risk between greater proteinuria and more adverse outcomes80;97;98, there is no clear cut-off. (Use of urinary protein quantification for prognostication in pre-eclampsia is discussed under ‘Prognosis of Pre-eclampsia’.) A threshold for low serum albumin of <20g/L has been used as the point at which edema develops from hypoproteinemia alone99-101.
Hyperuricaemia has not been included as an ‘adverse condition’, but was considered because of its association with pregnancy complications that is at least as strong as proteinuria80;102. To date, serum uric acid has not predicted adverse maternal outcomes in pre-eclampsia103.
Gestational age has not been listed as an ‘adverse condition’. However, onset of hypertension at <34 weeks is a risk marker for evolution of gestational hypertension to pre-eclampsia, and is associated with an increased risk of maternal and perinatal complications26;27;29;104-106.
Pre-eclampsia is not just hypertension
Understanding the pathogenesis of pre-eclampsia is key to understanding the multi-system and varied clinical manifestations of pre-eclampsia. The most popular theory for the pathogenesis of pre-eclampsia describes a two-stage process, which ultimately results in a mismatch between uteroplacental supply and fetal demands, leading to maternal endothelial cell dysfunction and the maternal (and fetal) manifestations of pre-eclampsia (Figure 1)107. For details, see the reviews by Roberts JM et al 108;109.
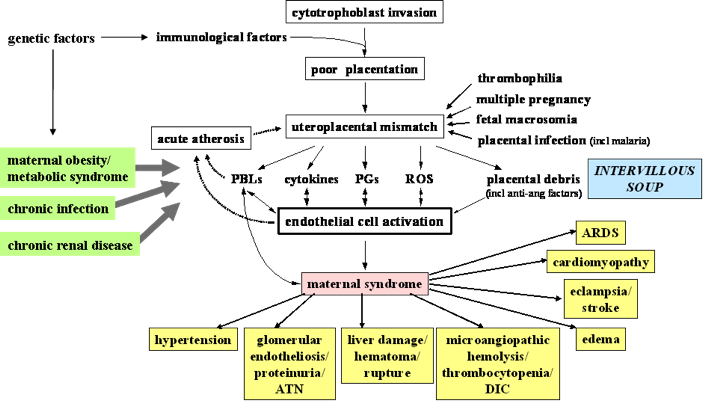
The most common maternal manifestations are those that are used to define pre-eclampsia clinically: hypertension and proteinuria. Other manifestations include visual scintiallations and scotomata that reflect occipital cortical ischemia, persistent headache that indicate cerebral ischemia and/or edema, epigastric or right upper quadrant pain that reflects capsular irritation secondary to hepatic necrosis and/or haematoma, and dyspnea and/or chest pain that indicate non-cardiogenic pulmonary edema. None of these is specific to pre-eclampsia.
There are a few specific comments that should be made about maternal signs. Stroke may occur at a systolic BP of 160mmHg or more, lower than previously thought110;111. Stroke, pulmonary edema and hepatic failure/rupture are the leading causes of maternal death in pre-eclampsia112. The sensitivity and specificity of complications are unknown for clonus or hyperreflexia (which is common in pregnancy). Jaundice is a late finding, reflecting disseminated intravascular coagulation (DIC) or another diagnosis (e.g., acute fatty liver of pregnancy). The seizures of eclampsia are usually isolated; when women have been imaged before and after eclampsia, CT or MRI studies have usually shown ischemia followed by edema113-119.
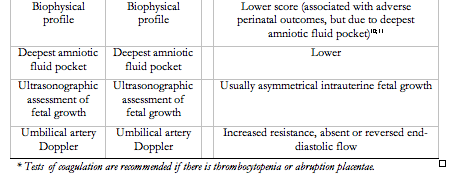
Fetal manifestations may occur with, precede, or occur in the absence of maternal manifestations120. The fetal syndrome consists of: oligohydramnios (i.e., low amniotic fluid), intrauterine fetal growth restriction, abnormal Doppler velocimetry of the umbilical artery [as measured by S/D (systolic/diastolic) ratio, pulsatility index or resistance index], decreased resistance to flow in the fetal middle cerebral artery (reflecting redistribution of blood flow to the central nervous system), an abnormal waveform in the ductus venosus, and/or stillbirth. Up to 30% of pre-eclampsia pregnancies are complicated by intrauterine fetal growth restriction, reflected by reduced fetal growth velocity121, and usually asymmetrical growth, although growth can be symmetrically reduced with severe placental disease or actually excessive122.
Investigations to classify the HDP #
Recommendations:
- For women with pre-existing hypertension , serum creatinine, serum potassium, and urinalysis should be performed early pregnancy if not previously documented (II-2, B).
- Among women with pre-existing hypertension, additional baseline laboratory testing may be based on other considerations deemed important by health care providers (III, C).
- Women with suspected pre-eclampsia should undergo the maternal laboratory (II-2, B) and fetal (II-1, B) testing as described in Table 2.
- If initial testing is reassuring, maternal and fetal testing should be repeated if there is ongoing concern about pre-eclampsia (e.g., change in maternal and/or fetal condition) (III, C).
- Uterine artery Doppler velocimetry can be used in hypertensive pregnant women to support a placental origin for hypertension, proteinuria, and/or ‘adverse conditions’ (II-2, B).
- Umbilical artery Doppler velocimetry can be used to support a placental origin for intrauterine fetal growth restriction (II-2, B).
Comments:
Pre-existing hypertension
Women with pre-existing hypertension will most likely (>95%) have essential hypertension, but secondary causes should be considered. A basic work-up has been suggested for women for whom suspicion of a secondary cause is low. (Please refer to the CHEP document for a more extensive discussion123. Additional baseline testing may be appropriate to diagnosis in early pregnancy, any associated problem(s) that may make difficult interpretation of bloodwork for pre-eclampsia end-organ dysfunction later in pregnancy; examples of such conditions include obesity and associated non-alcoholic steatohepatitis, or immune thrombocytopenia.
When pre-eclampsia is suspected
Women with suspected pre-eclampsia should undergo testing outlined in Table 2 to pick up end-organ dysfunction that is characteristic of this condition, or rule out important differential diagnoses (e.g., acute fatty liver of pregnancy). The validity of the various tests in Table 2, alone or in combination, has not been established. Uterine artery Doppler velocimetry may be useful in hypertensive pregnant women to support a placental origin for the hypertension, proteinuria, and/or ‘adverse conditions’124; obstetric consultation would then be warranted. Umbilical artery Doppler velocimetry may be useful. Absent or reversed end-diastolic flow in the umbilical artery, would be more consistent with placental dysfunction as a cause of IUGR, rather than decreased biological growth potential, uncertain dates, or aneuploidy125-128.
Pre-eclampsia may be a ‘disease in evolution’, with clinical manifestations unfolding in a serial fashion. When there is ongoing suspicion of pre-eclampsia, the nature of serial surveillance and its frequency are unclear, but a change in clinical status for mother or baby would be a reasonable indication.


Prediction, Prevention, and Prognosis of Pre-Eclampsia #
This section includes reccomendations, as well as comments about the prediction, prevention, and prognosis of Pre-Eclampsia
Predicting Pre-Eclampsia #
Recommendations:
- Women with markers of increased risk for pre-eclampsia (Shaded criteria, Table 3) should be offered obstetric consultation (II-2, B).
- Women at increased risk of pre-eclampsia should be considered for risk stratification involving a multivariable clinical and laboratory approach (II-2, B).
Comments:
There are many risk markers for pre-eclampsia, which include maternal demographics; past medical, obstetric, and family histories; and current pregnancy characteristics (Table 3). Many markers of pre-eclampsia risk are known at booking for antenatal care, and increase the risk of pre-eclampsia by two to four-fold129. These markers are shaded in grey in Table 3, and the strongest among them are previous pre-eclampsia and anti-phospholipid antibodies. For the other markers in Table 3, the strength of the association with pre-eclampsia is less well established or less consistent, or the marker pertains to information that becomes available in the second or third trimesters.
In the UK, the strongest clinical markers of pre-eclampsia risk that are identifiable at antenatal booking (i.e., those shaded in Table 3), have been recommended to screen for pre-eclampsia in the community (The pre-eclampsia community guidelines, PRECOG)130. It is recommended that women should be offered subspecialty referral if they have one of the bolded (and shaded markers), or two or more of the unbolded (and shaded markers) (grade D) (Table 3).
The markers of pre-eclampsia risk that become available in the second and third trimesters are based on the pathophysiological changes that characterize pre-eclampsia and in fact, precede clinical disease (i.e., after 20 weeks). Those risk markers that are best characterized are presented in Table 3. Many have been evaluated, and include measures of: placental perfusion and vascular resistance (e.g., mean second trimester BP, intravenous infusion of angiotensin-II, roll over test, 24-hr ambulatory BP monitoring, Doppler ultrasound); cardiac output and systemic vascular resistance; fetoplacental unit endocrinology (e.g., alpha fetoprotein, human chorionic gonadotropin); renal function (e.g., serum uric acid or microalbuminuria); endothelial function and endothelial-platelet interaction (e.g., platelet count, antiphospholipid antibodies, or homocysteine); oxidative stress (e.g., serum lipids); and circulating anti-angiogenic factors131;132. None of these (individually) have sufficient sensitivity and predictive values to be useful clinically, even among women at increased risk.
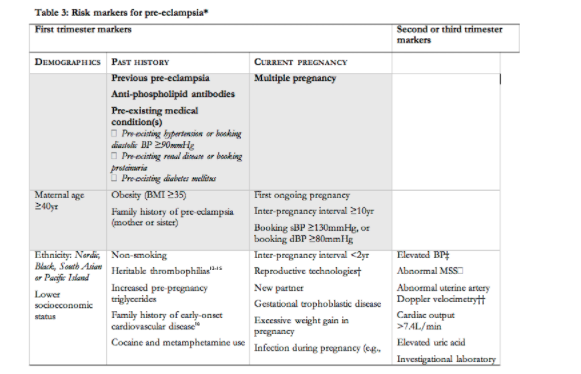
TABLE INCOMPLETE – requires footnotes
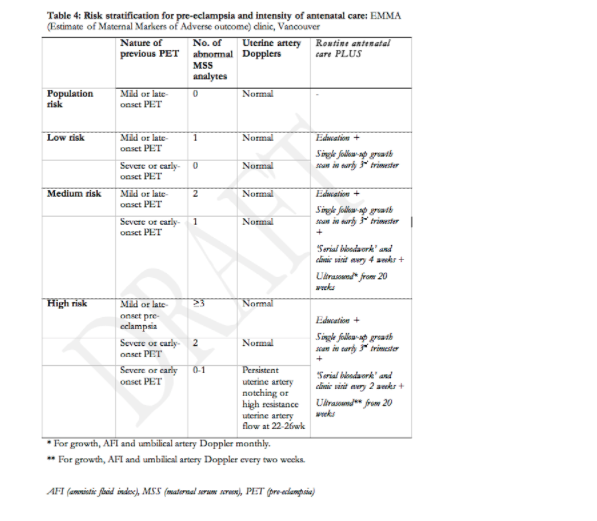
As there is no single test that predicts pre-eclampsia with sufficient accuracy to be clinically useful133, interest has grown in the development of multivariable models that include both clinical and laboratory predictors, available at booking and thereafter in pregnancy134. Women at increased risk of pre-eclampsia may benefit from this type of risk stratification. Table 4 presents an example of such a multivariable approach to risk stratification that distinguishes between population risk (5-7%), low risk (7-10%), intermediate risk (30-50%), and high risk (>60%) of pre-eclampsia in the current pregnancy so that antenatal care can be planned accordingly.
Preventing Pre-Eclampsia and its complications #
There is a considerable literature devoted to the prevention of pre-eclampsia. However, there is some controversy over whether or not prevention of pre-eclampsia per se is a worthy goal, rather than focussing on the prevention of the complications of pre-eclampsia. Non-severe gestational hypertension (or pre-eclampsia specifically) may have some adaptive function135. For example, neonatal morbidity is lower and neurodevelopmental outcome better among SGA babies whose mothers become hypertensive, as opposed to those who do not136. Therefore, we have based our recommendations on both the prevention of pre-eclampsia and/or its associated complications.
Based on PRECOG criteria, women are stratified, at booking, as being at low or increased risk of pre-eclampsia based on the presence (Table 3) of one of the bolded (and shaded) markers, or two or more of the unbolded (and shaded) markers (expert opinion).137 This approach, albeit arbitrary, does not recognize nulliparous women as requiring specialist consultation unless another risk marker for pre-eclampsia is present.
Preventing Pre-Eclampsia and its complications in women at LOW Risk #
Recommendations:
- Calcium supplementation (of at least 1g/d, orally) is recommended for women with low dietary intake of calcium (<600mg/d) (I, A).
- Although beneficial to promote and enhance maternal and fetal wellbeing, the patient should be counselled that the following do not prevent onset of preeclampsia: alcohol avoidance ( II-2, E), exercise (I, A), periconceptual folic acid supplementation (I, A), and smoking cessation (I, E).
- Although the following may be shown to be beneficial in reducing certain pregnancy complications, the patient should be counselled that the following do not prevent onset of preeclampsia: prostaglandin precursors (I, C), magnesium supplementation (I, C), and zinc supplementation (I, C).
- The patient should be counselled that the following are not recommended in preventing preeclampsia: dietary salt restriction (I, D), calorie restriction during pregnancy in overweight women (I, D), low dose aspirin (I, E), Vit C and E supplementation (I, E), or thiazide diuretics ( I, E).
Comments:
There are a number of issues that should be adressed when looking at preventing pre-Eclampsia and its complications in low risk women. These include Alcohol avoidance, Calcium, Dietary changes, Folate-containing multivitamins, Lifestyle changes, Micronutrients other than calcium, Prostaglandin precursors, Smoking cessation, Thiazide diuretics, Vitamins C and E, and Other interventions for which no recommendation can be made.
Alcohol Avoidance and Aspirin (Low Dose) #
Alcohol avoidance
There are no trials on the impact of alcohol avoidance on the incidence of HDPs, although reduced consumption is recommended to reduce BP in non-pregnant individuals138. There is no proven safe level of alcohol consumption in pregnancy [Alcohol,nitcotine, substance use; Motherisk Program, March 14, 2007; http://www.motherisk.org/prof/alcohol.jsp].
Aspirin (low-dose)
Low dose aspirin does not decrease the incidence of pre-eclampsia in low risk nulliparous women (RR 0.93, 95% CI 0.81, 1.08)139-143.
Calcium #
There is an inverse relationship between dietary calcium intake and BP in the general population144. Low calcium intake (<600mg/day, corresponding to less than two dairy servings per day) may be harmful by causing vasoconstriction, either through increasing magnesium levels, or by stimulating release of parathyroid hormone or renin, thereby increasing vascular smooth muscle intracellular calcium145. Oral calcium supplementation (of at least 1g/d) decreases the incidence of pre-eclampsia [RR 0.68, 95% CI 0.49, 0.94) (7 trials including the American CPEP trial146, 14,619 women], due to a small decrease among women with low calcium intake (RR 0.81, 95% CI 0.67, 0.99) (4 trials, 9,775 women)144. Maternal death or serious morbidity was also reduced (RR 0.80, 95% CI 0.65, 0.97) (1 trial, 8,312 women)147. The use of calcium supplementation may have been discouraged by the results of the largest (low-risk) CPEP trial, in which calcium supplementation was not effective in a low-risk, nulliparous population with adequate dietary calcium148. There were no documented adverse effects of calcium supplementation144. An alternative to supplementation may be an increase in dietary calcium intake, by 3-4 dairy servings per day (as one serving corresponds to 250-300mg of calcium).
Dietary Changes #
Dietary salt restriction (with confirmed compliance) does not affect the incidence of gestational hypertension or pre-eclampsia specifically (RR 1.11, 95% CI 0.46, 2.66) (2 trials, 603 women)149.
A heart-healthy diet has been associated with a lower risk of pre-eclampsia in a case-control study150. No trials of this intervention were identified.
Energy or protein restriction for women who are overweight, or those with excessive weight gain in pregnancy, did not result in a decreased incidence of pre-eclampsia or gestational hypertension (3 trials, 384 women)151. There are theoretical concerns about the impact of starvation ketosis on fetal neurodevelopment152.
Folate-containing multivitamins #
Periconceptual use of a folate-containing multivitamin is recommended for all women, for primary prevention of neural tube and possibly, other congenital anomalies including cardiovascular and limb defects153. Periconceptual and ongoing regular use of multivitamins has been associated with primary prevention of gestational hypertension (1 trial, 138 women)154 and pre-eclampsia in women with a body mass index <25 (prospective cohort, 1,835 women)155. (Use of vitamins C and E for women at increased risk of pre-eclampsia will be discussed later in this document.)
Lifestyle changes #
Observational studies have associated exercise (and greater intensity of exercise) with a reduced risk of pre-eclampsia156-162. Potential mechanisms include a decrease in BP, lipids, and proinflammatory cytokines163. We were unable to identify trials of exercise for pre-eclampisa prevention among women at low risk. However, exercise of low to moderate intensity is beneficial for general health reasons to maintain or improve physical fitness (11 trials, 472 women)164. Overweight women who exercised from early pregnancy had improved exercise capacity without demonstrated differences in substantive clinical outcomes (1 trial, 132 women)165.
Pre-eclampsia is associated with greater workload166;167 and stress168, even among women at low risk, but the quality of the evidence does not allow for firm conclusions169. Although workload reduction is a common obstetric intervention, we were unable to identify randomized studies of workload or stress reduction on the incidence of pre-eclampsia. These are unlikely to be forthcoming given the nature of the interventions.
Micronutrients other than calcium #
Micronutrient deficiencies other than calcium are often found in pregnancy, but those at risk are difficult to identify clinically. Deficiencies of magnesium, zinc and pyridoxine have been associated with an increase in HDP and/or their complications170-172.
Magnesium is an essential mineral involved in protein synthesis and electrical potentials of muscle membranes and nerves. Magnesium supplementation (various preparations) in primarily low-risk women did not affect the incidence of a HDP, but did decrease preterm birth (RR 0.73, 95% CI 0.57, 0.94), low birthweight (RR 0.67, 95% CI 0.46, 0.96), and SGA infants (RR 0.70, 95% CI 0.53, 0.93) (7 trials, 2,689 women)171. However, no conclusions can be drawn because only one included trial was of high quality.
Zinc plays a critical role in protein synthesis and nucleic acid metabolism. Zinc supplementation (20-90mg elemental zinc) in primarily low-risk women did not affect the incidence of a HDP, although decreases in preterm delivery (RR 0.73, 95% CI 0.54, 0.98) and Caesarean section (RR 0.69, 95% CI 0.49, 0.96) reached statistical significance (7 trials, 1,962 women)170.
Prostaglandin precursors #
Diets rich in marine oils are associated with a reduced risk of pre-eclampsia173. Such marine and other oils (e.g., evening primrose oil) are rich in prostaglandin precursors and may be beneficial by reducing inflammation and vasoconstriction. These oils (referred to as ‘prostaglandin precursors’ for brevity) did not decrease the risk of pre-eclampsia in mixed populations that included both low and high risk women (RR 0.86, 95% CI 0.59, 1.27) (6 trials, 2,783 women)173. However, birth before 34 weeks was marginally decreased (RR of 0.69, 95% CI 0.49, 0.99). Although marine oil supplementation may be useful, increased dietary intake of fish for the purpose of fish oil consumption, is not recommended because of concerns about contaminants, such as mercury174.
Smoking cessation and Thiazide diuretics #
Smoking Cessation
Smoking is associated with a reduced risk of pre-eclampsia in observational studies. However, smoking cessation is recommended to decrease low birthweight (RR 0.81, 95% CI 0.70, 0.94) and preterm birth (RR 0.84, 95% CI 0.72, 0.98) (57 trials, 28,431 women)175. Various approaches have been tried. An ongoing RCT is evaluating the effectiveness and safety of nicotine replacement therapy in pregnancy176.
Thiazide diuretics
Thiazide diuretics did not decrease pre-eclampsia (RR 0.68, 95% CI 0.45, 1.03) or other substantive outcomes in women at low risk of pre-eclampsia (5 trials, 1,836 women)177. Maternal side effects were more common than among women who took placebo, but there was no increase in any other substantive adverse maternal or perinatal outcome.
Pre-eclampsia is associated with oxidative stress. However, in an adequately powered RCT of vitamins C (1000 mg/d) and E (400 IU/d) in low-risk nulliparous women, vitamins C and E therapy from 14-22 weeks showed no reduction in the incidence of pre-eclampsia (1 trial, 1,877 women)178. In a secondary analysis of these data, vitamins C and E actually increased the incidence of pre-eclampsia defined as gestational hypertension with proteinuria. The (low risk arm of the) INTAPP trial of vitamins C and E before 18 weeks was stopped early but data are pending (www.obstgyn.ca/mfmresearch/INTAPP). The NIH CAPPS Trial of vitamins C and E from 9-16 weeks in low risk primigravida is ongoing (www.nichd.nih.gov/womenshealth/research/pregbirth/disorders.cfm).
Other interventions for which no recommendation can be made #
Interest in supplementation with iron and/or folate (beyond 10 weeks’ gestation) stems from the importance of anaemia in developing countries and further progressive anaemia associated with pregnancy. There is insufficient evidence on which to comment on the impact on pre-eclampsia of either routine (vs. no routine) iron supplementation (usually 60-100mg elemental iron/day) on pre-eclampsia (1 trial, 47 women) or routine iron with/without folic acid supplementation (1 trial, 48 women)179.
Pyridoxine has many roles including neurological development and function. Although pyridoxine supplementation did not decrease the risk of pre-eclampsia (in 5 trials, 1,646 women), the trials were of poor quality with poor reporting of substantive outcomes, making conclusions impossible to draw172.
We were unable to identify trials administering the following agents for primary prevention of pre-eclampsia: garlic, vitamin A, selenium, copper or iodine.
Preventing Pre-Eclampsia and its complications in women at INCREASED Risk #
Prevention of pre-eclampsia has been extensively studied in women at ‘increased risk’, defined most commonly as: maternal age <18 years, positive roll-over test (reflecting increased sensitivity to angiotensin-II but not longer performed clinically), multiple pregnancy, pre-existing hypertension and/or previous pre-eclampsia.
Recommendations:
- The following should be recommended for women at increased risk : low-dose aspirin (I, A), and calcium supplementation (of at least 1g/d) for women with low calcium intake (I, A);
- Low-dose aspirin should be given in a dose of 75-100mg/d (III, B), administered at bedtime (I, B), started pre-pregnancy or from diagnosis of pregnancy but before 16 weeks’ gestation (III, B), and continued until delivery (I, A).
- The following may be advised to reduce the risk of developing preeclampsia in women at increased risk: avoidance of inter-pregnancy weight gain (II-2, E), increased rest at home in the third trimester (I, C), and reduction of workload or stress (III, C).
- Although useful in promoting maternal and fetal well being, the patient at increased risk should be counselled that the following do not prevent preeclampsia: alcohol avoidance (II-2, E), periconceptual use of a folate containing multivitamin ( I, A), and smoking cessation (I, E)
- Although useful in preventing certain pregnancy complications, the patient at increased risk should be counselled that the following do not prevent preeclampsia: prostaglandin precursors (I, C); or magnesium supplementation (I, C).
- The patient at increased risk should be counselled that the following The following are not recommended to prevent preeclampsia: dietary salt restriction during pregnancy (I, D); calorie restriction in overweight women during pregnancy (I, D); weight maintenance in obese women during pregnancy (III, D); antihypertensive therapy specifically to prevent pre-eclampsia (I, D), vitamins C and E supplementation (I, E),
Comments:
- The following may be advised to reduce the risk of developing preeclampsia in women at increased risk: avoidance of inter-pregnancy weight gain (II-2, E), increased rest at home in the third trimester (I, C), and reduction of workload or stress (III, C).
- Although useful in promoting maternal and fetal well being, the patient at increased risk should be counselled that the following do not prevent preeclampsia: alcohol avoidance (II-2, E), periconceptual use of a folate containing multivitamin ( I, A), and smoking cessation (I, E)
- Although useful in preventing certain pregnancy complications, the patient at increased risk should be counselled that the following do not prevent preeclampsia:The following are not recommended for pre-eclampsia prevention, but may be useful for prevention of other pregnancy complications: prostaglandin precursors (I, C); or magnesium supplementation (I, C).
- The patient at increased risk should be counselled that the following The following are not recommended to prevent preeclampsia: dietary salt restriction during pregnancy (I, D); calorie restriction in overweight women during pregnancy (I, D); weight maintenance in obese women during pregnancy (III, D); antihypertensive therapy specifically to prevent pre-eclampsia (I, D), vitamins C and E supplementation (I, E),
Antihypertensive therapy does not prevent pre-eclampsia (RR 0.99, 95% CI 0.84, 1.18) or the associated adverse perinatal outcomes, but among women with mild hypertension, severe hypertension is halved in incidence (RR 0.52, 95% CI 0.41, 0.64) (24 trials, 2,815 women)180. Antihypertensive therapy cannot be recommended for pre-eclampsia prevention until it can be demonstrated that the decrease in maternal blood pressure is not outweighed by a negative impact on perinatal outcomes29;181;182. (Antihypertensive therapy for treatment of elevated BP is discussed under ‘Management’.)
As with Low risk, when when attempting to prevent pre-eclampsia and its complications in women at increased risk, there are a number of issues that should be looked at. These include Aspirin (low-dose), Calcium, Dietary changes, Folate-containing multivitamin, Lifestyle changes, Micronutrients other than calcium, Prostaglandin precursors, and Vitamins C and E.
Aspirin (low-dose) #
In women at increased risk of pre-eclampsia, low-dose aspirin results in a small decrease in: pre-eclampsia (RR 0.85, 95% CI 0.78, 0.92; NNT 69, 95% CI 51, 109 women; 43 trials, 33,439 women for this outcome), preterm delivery (RR 0.92, 95% CI 0.88, 0.97; NNT 83, 95% CI 50, 238 women), and perinatal death (RR 0.86, 95% CI 0.75, 0.98; NNT 227, 95% CI 128, 909 women) (51 trials, 36,500 women overall)183. Findings are similar when individual patient data are subjected to detailed analysis (31 trials, 32,217 women); although the decrease in perinatal death no longer reaches statistical significance (RR 0.91, 95% CI 0.81, 1.03), the incidence of serious adverse maternal/perinatal outcome is decreased by aspirin (RR 0.90, 95% CI 0.85, 0.96)184. There is no evidence of short or long-term adverse effects on the mother or newborn. Aspirin does not increase miscarriage risk185.
Who should receive aspirin and in what dose is unclear. There are no clear demonstratable benefits based on historical risk markers (such as pre-existing hypertension) or details of aspirin administration (such as aspirin dose). Subgroup analyses in meta-analyses of aspirin trials appear to indicate that aspirin may be more effective for women at greatest baseline risk, when started before 16 weeks’ gestation, and when aspirin is used at a higher dose183;186-188. This may be because some women may be more resistant to the effects of aspirin189, and/or a dose of at least 75mg/d may be necessary to inhibit both platelet and placental thromboxane. However, a dose of 100mg/d may affect fetal prostacyclin synthesis190. One RCT found that taking aspirin at bedtime resulted in lower BP than taking aspirin in the morning183;191. Aspirin may be continued until delivery192 (Please see ‘Anaesthesia and fluid administration’).
Calcium and Dietary Changes #
Calcium
Oral calcium supplementation (of at least 1g/d) decreases the incidence of pre-eclampsia (RR 0.22, 95% CI 0.12, 0.42) and preterm delivery (RR 0.45, 95% CI 0.24, 0.83) (5 trials, 587 women)144. Three trials were conducted in low calcium intake populations. No trial included women with previous pre-eclampsia. There were no documented adverse effects of calcium supplementation. An alternative to supplementation may be an increase in dietary calcium intake, by 3-4 dairy servings per day (as one serving corresponds to 250-300mg of calcium).
Dietary changes
We were unable to identify trials of dietary salt restriction on the incidence of pre-eclampsia among women at increased risk.
We were unable to identify trials of a heart-healthy diet for pre-eclampsia prevention. Women with pre-existing hypertension who are already following a DASH diet may continue this diet during pregnancy but there is no evidence to support this practice.
Obesity is both a major public health problem and a risk marker for pre-eclampsia. No effect on gestational hypertension (or pre-eclampsia specifically) has been demonstrated when overweight women have received dietary counselling during pregnancy to curb the rate of weight gain (3 trials, 384 women)151. No trials have addressed the impact of pre-pregnancy or early pregnancy weight reduction on pre-eclampsia; there are theoretical concerns about the impact of starvation ketosis on fetal neurodevelopment193.
Folate-containing multivitamin #
Periconceptual and ongoing regular use of multivitamins was associated with higher birthweight centiles in a secondary analysis of the VIP (vitamin C and E trial) in the UK194. Periconceptual use of a folate-containing multivitamin is recommended for all women of child-bearing age for prevention of neural tube, and possibly, other birth defects (I, A).
Enthusiasm for the use of heparin to prevent pre-eclampsia and other adverse placental complications comes from the effective use of unfractionated heparin for women with antiphospholipid syndrome and recurrent pregnancy loss195. It is unclear whether or not heparin does more harm than good for women with a history of pre-eclampsia, even in the setting of an inherited or acquired thrombophilia. There are no completed trials of heparin for pre-eclampsia prevention in women with thrombophilia196. The only trial in non-thrombophilia women enrolled a special population of non-thrombophilic women with the angiotensin-converting enzyme DD polymorphism (80 women), LMWH (dalteparin 5,000IU/d) decreased pre-eclampsia recurrence by 75%197. Potential benefits of thromboprophylaxis must be weighed against the cost, inconvenience and possible side effects of treatment. Practitioners are encouraged to enrol their patients in clinical trials (e.g., TIPPS {www.ohri.ca/programs/clinical_epidemiology/Thrombosis_Group/studies/ TIPPS.asp}).
Lifestyle changes #
There is robust epidemiological data that weight gain between pregnancies (even in non-obese women) is associated with significantly more pre-eclampsia and other pregnancy complications, such as Caesarean section and gestational diabetes198.
Physical activity is associated with a reduced incidence of pre-eclampsia199;200. No impact of exercise was seen on gestational hypertension or pre-eclampsia (2 trials, 45 women)199; there is one ongoing high quality of trial of moderate intensity exercise in women with previous pre-eclampsia201. In women at increased risk of pre-eclampsia, it is not known whether exercise (to improve or maintain fitness) is of greater benefit than risk.
Physically demanding work is associated with a higher risk of gestational hypertension and pre-eclampsia [OR 1.60, 95% CI 1.30, 1.96; 4 observational studies, 5,837 women]202. Although workload reduction is a common obstetric intervention, we were unable to identify randomized studies of workload or stress reduction on the incidence of pre-eclampsia. These are unlikely to be forthcoming given the nature of the interventions.
Increased rest at home (varying from 30 minutes to 6 hours/day) in the third trimester of pregnancy decreased the incidence of pre-eclampsia (RR 0.05, 95% CI 0.00, 0.83 for increased rest alone; RR 0.13, 95% CI 0.03, 0.51 for rest plus a nutrient supplement) (2 trials, 106 women)203. Other substantive outcomes (such as adverse effects of rest and women’s views) were not reported. There is a lack of clarity about the definition of bed rest, and whether women comply with activity restriction204.
Micronutrients other than calcium #
Magnesium supplementation (various preparations) administered to a mixed population of low and high risk women in (7 trials, 2,689 women) did not decrease the risk of pre-eclampsia, but decreases were seen in: preterm birth (RR 0.73, 95% CI 0.57, 0.94), low birth weight (RR 0.67, 95% CI 0.46, 0.96), and small for gestational age infants (RR 0.70, 95% CI 0.53, 0.93)171. However, no conclusions can be drawn because only one included trial was of high quality.
Selenium supplementation in the third trimester was reported to decrease “gestational hypertension”, but this was not defined (1 trial, 100 women)205.
Garlic may decrease lipid peroxidation and platelet aggregation. In a small trial of 100 women at increased risk of pre-eclampsia based on a positive roll-over test, no impact of garlic was seen on “pre-eclampsia”; garlic supplementation was association with more reports of “odour” than was placebo206.
We did not identify trials of zinc, pyridoxine, iron (with/without folic acid), zinc, multivitamins with/without micronutrients, vitamin A, iodine or copper for pre-eclampsia prevention in women at increased risk.
Prostaglandin precursors and Vitamins C and E #
Prostaglandin precursors
Prostaglandin precursors did not decrease the risk of pre-eclampsia in mixed population of low and high risk women (RR 0.87, 95% CI 0.59, 1.28) (5 trials, 1,683 women)173. Birth before 34 weeks was marginally decreased (RR 0.69, 95% CI 0.49, 0.99).
Vitamins C and E
Vitamins C (1000 mg/d) and E (400 IU/d) decreased the risk of pre-eclampsia in one207 of two small pilot RCTs (2 trials, 483 women)207;208. Another small RCT found a decreased risk of pre-eclampsia with administration of multiple anti-oxidants (including vitamins C and E) in women who had a low superoxidedismutase levels (1 trial, 60 women)209. However, in an adequately powered RCT in high-risk women (VIP Trial210), vitamins C and E did not decrease the incidence of pre-eclampsia; rather, vitamins C and E were more frequently associated with birthweight <2.5 kg211. The (high risk arm of the) INTAPP trial of vitamins C and E before 18 weeks in women at increased risk of pre-eclampsia was stopped early but data are pending (www.obstgyn.ca/mfmresearch/INTAPP).
Prognosis (Maternal and Fetal) in Pre-Eclampsia #
Recommendations:
- Serial surveillance of maternal well-being is recommended, both antenatally and postpartum (Table 2) (II-3, B)
- The frequency of maternal surveillance should be at least once per week antenatally, and at least once in the first three days postpartum (III, C).
- Serial surveillance of fetal well-being is recommended (II-2, B).
- Antenatal fetal surveillance should include umbilical artery Doppler velocimetry (I, A).
- When gestational hypertension with neither proteinuria nor ‘adverse conditions’ develops before 34 weeks, these women should be followed closely for maternal and perinatal complications (II-2, B).
Comments:
Women with pre-eclampsia should undergo serial maternal and fetal surveillance of well-being. However, the nature of surveillance (and its frequency), particularly among women undergoing expectant management of pre-eclampsia, has not been defined. Table 2 presents a list of suggested investigations, based on detection of end-organ dysfunction. A comprehensive programme of maternal and fetal evaluation (that included all of the tests recommended in Table 2) decreased adverse maternal outcomes from 5.1% to 1.2% in one tertiary perinatal centre212. Maternal surveillance should continue postpartum because of the risk of postpartum deterioration, particularly when there are end-organ complications of pre-eclampsia213.
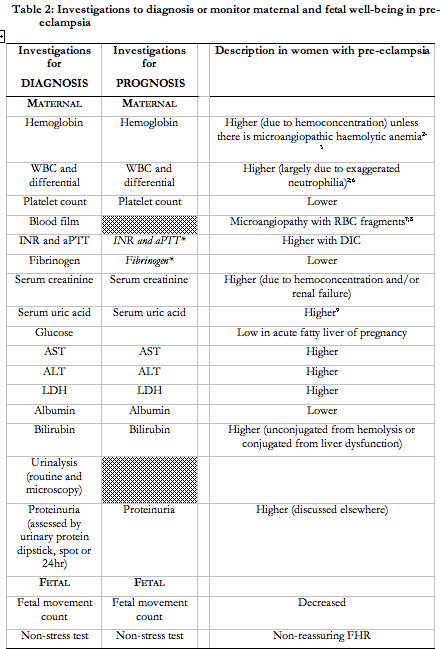
Maternal surveillance
In a 1999 survey, at least 80% of Canadian obstetric care providers reported using: complete blood count; coagulation tests; serum creatinine; serum uric acid; aspartate and alanine aminotransferases; lactate dehydrogenase; urinary dipstick proteinuria; and 24hr urinary protein214. These were performed at least once/week (and rarely daily).
Among women with proteinuria, higher (vs. lower) levels of proteinuria have not been consistently associated with higher maternal or perinatal mortality or morbidity215-219, and have not predicted short term maternal renal failure or ongoing proteinuria220-223. However, given the central role of proteinuria in pre-eclampsia, we are unwilling to recommend against use of protein quantification (by any method) until further data are available.
Fetal surveillance
In general, a programme of antepartum fetal assessment reduces perinatal morbidity and/or mortality in women with HDP (www.sogc.org/guidelines, 2000). In general, few trials have compared these techniques and no one technique appears to be superior. For gestational hypertension or pre-eclampsia specifically, use of umbilical artery Doppler velocimetry appears to decrease perinatal mortality (OR 0.71, 95% CI 0.50, 1.01) (11 trials, nearly 7,000 women)224 (www.sogc.org/guidelines, 2007). Weekly Doppler interrogation of the umbilical artery is suggested as reasonable clinical practice.
In the same 1999 survey mentioned under ‘Maternal surveillance’ (above), at least 80% of Canadian obstetricians reported using: kick count; non-stress test/cardiotocography; and biophysical profile214. Compared with maternal surveillance, there is less consistency regarding frequency of fetal testing: daily kick counts daily (83%); at least weekly NST (65%), BPP (88%), or umbilical artery Doppler velocimetry (56%); and less than once weekly ultrasonographically estimated fetal weight.
Gestational hypertension
Approximately 35% of women with gestational hypertension with onset at <34 weeks evolve into pre-eclampsia26;27;29;225-227, and the associated risks of serious maternal (2%) and perinatal complications (16%) are high228. These women should receive heightened maternal and fetal surveillance, the nature and frequency of which has not been established
Treatment of the Hypertensive disorders of Pregnancy (HDP) #
In the treatment of hypertensive disorders of pregnancy (HDP), there is both Antenatal treatment and Postpartum treatment.
Antenatal Treatment #
Antenatal treatment has been divided into 9 sections: dietary changes, lifestyle changes, Place of care, antihypertensive therapy, Corticosteroids for acceleration of fetal pulmonary maturity, mode of delivery, anaesthesia including fluid administration, aspects of care specific to women with pre-existing hypertension, and aspects of care specific to women with pre-eclampsia.
Dietary Changes #
Recommendations:
- For the purpose of improved blood pressure control, new dietary salt restriction is not recommended (II-2, D).
- There is insufficient evidence to make a recommendation about the usefulness of: ongoing salt restriction among women with pre-existing hypertension (III, I); heart-healthy diet (III, I), or calorie restriction for obese women (III, I). commets in the tex belowt on this suffices
Comments:
We were unable to identify trials examining the impact of the following on outcomes in any of the HDP: new salt restriction, ongoing salt restriction among women with pre-existing hypertension, heart-healthy diet, or calorie restriction among women who are overweight. An observational study did find that for pre-eclampsia, a low-salt diet did not decrease BP but did accelerate volume depletion which may be of theoretical harm229.
Lifestyle changes #
Recommendations:
- For the purpose of enhanced blood pressure control, the patient should be counselled that there is currently insufficient evidence to make a recommendation about the usefulness of: exercise, workload reduction, or stress reduction (all III, I).
- For women with gestational hypertension (without pre-eclampsia), some bed rest in hospital (compared with unrestricted activity at home) may be useful (I, B).
- For women with pre-eclampsia who are hospitalized, strict bed rest is not recommended (I, D).
Comments:
We were unable to identify studies of the impact of exercise on outcomes among a HDP. However, pre-eclampsia is listed as a contraindication to vigorous exercise in the SOGC 2003 Clinical Practice Guidelines on Exercise in Pregnancy [www.sogc.org/guidelines].
It is common practice to recommend workload reduction or cessation when either non-severe GH or pre-eclampsia are diagnosed and outpatient care is continued. There are no RCT data to support this practice, although it may be practical from the perspectives of both patient (e.g., facilitating maternal and fetal monitoring) and employer (e.g., transition planning). Outside pregnancy, stress management may be useful if stress appears to be associated with hypertension.
Since its introduction in 1952230, bed rest has become standard therapy for women with a HDP, as either primary or adjunctive therapy231. How bed rest is defined has varied widely, and compliance with recommendations has been further questioned232. However, bed rest should be clearly beneficial before it can be recommended, in hospital or at home, because bed rest may have harmful physical, psychosocial and financial effects233;234. There is limited RCT evidence to consider.
For pre-eclampsia (defined as gestational hypertension with proteinuria), strict (vs. some) bed rest in hospital is not associated with differences in maternal or perinatal outcomes (2 trials, 145 women) (Crowther CA 1986, cited in231)235. For gestational hypertension (without pre-eclampsia), some bed rest in hospital (vs. routine activity at home) decreases severe hypertension (RR 0.58, 95% CI 0.38, 0.89) and preterm birth (RR 0.53, 95% CI 0.29, 0.99) (2 trials, 304 women), although women prefer unrestricted activity at home236-238; whether the beneficial effect is from the bed rest or the hospitalization is not clear.
Place of care #
Recommendations:
- Inpatient care should be provided for women with severe hypertension or severe pre-eclampsia (II-2, B).
- A component of care through hospital day units (I, B) or as home care (II-2, B) can be considered for women with non-severe pre-eclampsia or non-severe (pre-existing or gestational) hypertension.
Comments:
Out-of-hospital care for pre-eclampsia assumes that a full assessment (usually in hospital) of maternal and fetal well-being has been made, and that women do not have severe disease (see Classification of the HDP). The literature has excluded women with severe hypertension or severe pre-eclampsia. The options for outpatient care include: obstetrical day units and home care (usually through formal antepartum home care programmes). Eligibility will depend on the woman’s location of primary residence from the hospital, ability to provide adequate surveillance, patient compliance, lability of BP, and lack of progression of their pre-eclampsia or co-morbid conditions.
Hospital day units
Eligibility has varied from 30-60%239;240. Trials have focussed on gestational hypertension, and compared care in hospital day units to inpatient care (2 trials, 449 women)240;241. Maternal and perinatal outcomes and costs were similar, although days in hospital were reduced by care in day units. Women preferred out-of-hospital care in trials240 and in previous observational studies242.
Home care
Eligibility for formal home care programmes is no greater than 25%243, although eligibility criteria have varied widely. As a basis for home care, it has been shown that women can accurately measure BP at home using an automated device244, and that BP at home is not consistently different from that in hospital, although values for individual women vary widely, particularly for those on antihypertensive therapy245.
In observational studies, the definition of ‘home care’ has varied in terms of: prescriptions for bed rest; proportion of self-assessments vs. those done by a nurse/midwife; and communication in person, by telephone conversation, or by telephonic electronic transfer246;247. However, all involved some component of daily contact and a (usually) weekly hospital or office outpatient visit246-248.
No RCTs have compared a formal antepartum home care programme with either hospital day care or inpatient care. However, for gestational hypertension (without pre-eclampsia), routine activity at home (vs. some bed rest in hospital) is associated with more severe hypertension (RR 1.72, 95% CI 1.12, 2.63) and preterm birth (RR 1.89, 95% CI 1.01, 3.45) (2 trials, 304 women), but women prefer routine activity at home236;237. It is unclear whether the beneficial effect of bed rest in hospital is from the bed rest or the hospitalization. Formal antepartum home care programmes include some component of bed rest.
In observational studies of antepartum home care (vs. inpatient care), hospital admission (25%)247 and re-admission rates (44%)249 were quite high. However, home care resulted in similar maternal and perinatal outcomes among women with mild pre-eclampsia (321 women)250 or gestational hypertension (592 women)251, and reduced costs247. Women were satisfied with home care252.
Antihypertensive therapy #
The following recommendations apply to women with either pre-existing or gestational hypertension.
For severe hypertension (BP of >160mmHg systolic or ≥110mmHg diastolic)
Recommendations:
- The goal of BP treatment should be to lower the blood pressure lowered to <160mmHg systolic and <110mmHg diastolic (II-2, B).
- Initial antihypertensive therapy should be with: labetalol (I, A); nifedipine capsules (I, A); nifedipine PA tablets (I, B); or hydralazine (I, A) (Table 5).
- MgSO4 while used to prevent seizures, is not recommended as an antihypertensive agent (I, E).
- In hospitalized women who are undergoing aggressive therapy to lower a high, unstable blood pressure, continuous FHR monitoring is advised until BP is stable (III, I).
- Nifedipine and MgSO4 can be used contemporaneously (II-2, B).
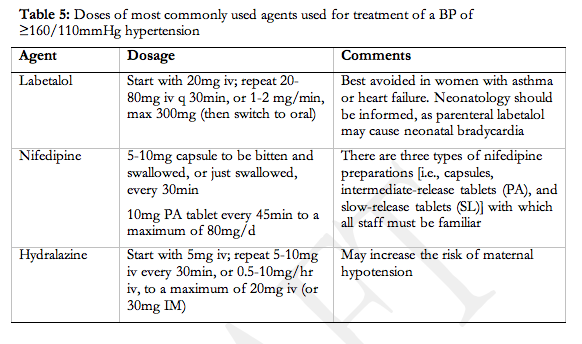
Comments:
Severe elevations of BP (i.e., ≥160/110mmHg) should be confirmed, after 15min. There is general consensus that severe hypertension should be treated in pregnancy to decrease maternal morbidity and mortality253. Most women with severe hypertension in pregnancy will have pre-eclampsia, and most of those will have had normal BP in the recent past. These hypertensive events are considered ‘urgencies’, given such potentially large and acute increases in BP, even in the absence of symptoms.
Obstetricians most frequently prescribe parenteral hydralazine or labetalol for treatment of severe hypertension, based on a 1999 survey of Canadian practitioners254. By meta-analysis of the relevant (21 trials, 1,085 women), parenteral hydralazine (compared with other short-acting antihypertensives) may be associated with more adverse effects, including maternal hypotension, Caesarean section, and adverse FHR effects255. Observational literature illustrates that hypotension may result with any short-acting antihypertensive agent administered to women with pre-eclampsia, because they are intravascularly volume depleted. Therefore, it may be prudent to continuously monitor FHR until BP has stabilized. From the same meta-analysis, labetalol may associated with more neonatal bradycardia (which required intervention in one of six affected babies)256;257. Labetalol was administered parenterally; however, it has been given orally for hypertensive urgencies, with good effect258.
Forty percent of Canadian obstetricians described frequent use of MgSO4 for treatment of severe hypertension254. The limited (and observational) literature describes no259 or transient decreases in BP260-263, 30min after 2-5g of iv MgSO4 (with/without ongoing infusion), usually in the setting of pre-eclampsia. Therefore, although a sustained lowering of BP cannot be anticipated following a MgSO4 bolus, the potential for a transient lowering of BP 30min after administration should be considered when antihypertensives are co-administered.
The nifedipine preparations that are appropriate for treatment of severe hypertension are the capsule and the PA tablet264. Most authors of randomized trials did not specify whether nifedipine capsules were bitten (prior to swallowing), which may have a greater effect on BP. Although the 5mg (vs. 10mg) capsule may reduce the risk of a precipitous fall in BP, there are no published studies comparing the 5mg and 10mg doses. The risk of neuromuscular blockade with contemporaneous use of nifedipine and MgSO4 is <1%, based on a single-centre, controlled study, and a complete data synthesis from the literature265; blockade is reversed with 10g of iv calcium gluconate.
NTG is primarily venodilatory. Theoretically, it may not be a good choice of antihypertensive in women with pre-eclampsia. However, no adverse clinical effects have been demonstrated in small studies266;267. For refractory hypertension, in an intensive care setting, consideration can be given to using sodium nitroprusside or diazoxide268.
For non-severe hypertension (BP of 140-159/90-109mmHg)
Recommendations:
- For women without co-morbid conditions , antihypertensive drug therapy should be used to keep sBP at 130-155mmHg and dBP at 80-105mmHg (III, C).
- For women with co-morbid conditions (Table 1), antihypertensive drug therapy should be used to keep sBP at 130-139mmHg and dBP at 80-89mmHg (III, C).
- Initial therapy can be with one of a variety of antihypertensive agents available in Canada: methyldopa (I, A), labetalol (I, A), other beta-blockers (acebutolol, metoprolol, pindolol, and propranolol) (I, B), and calcium channel blockers (nifedipine) (I, A).
- Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) should not be used (II-2, E).
- Atenolol and prazosin are not recommended in the treatment of HDP (I, D).
Comments:
How to manage a BP of 140-159/90-109 is much debated. Any antihypertensive therapy will, compared with placebo or no therapy, decrease the risk of transient, severe hypertension (RR 0.50, 95% CI 0.41 to 0.61; 19 trials, 2,409 women; NNT 9-17), without a clear difference in other maternal or perinatal outcomes, such as stroke, perinatal death or preterm delivery (28 trials, 3,200 women)269;269. Antihypertensive therapy may be harmful, based on the results of a small pilot RCT29;182 and a meta-regression of RCTs which found a significant relationship between the antihypertensive-induced fall in mean arterial pressure and the risk of SGA infants or lower birthweight270;271. There are no reliable data on long-term developmental outcomes272;273. A large definitive trial is needed.
Women without co-morbid conditions should have antihypertensive therapy to lower dBP to 80-105mmHg. Choosing an upper limit of 105mmHg for dBP acknowledges intra-patient variability in BP, the inaccuracies of BP measurement, the desire to avoid severe diastolic hypertension (dBP ≥110mmHg), and the recognition that outside of pregnancy, non-severe hypertension is not an indication for immediate treatment274. Choosing a lower limit of 80mmHg for the dBP goal is consistent with non-pregnancy recommendations that do not recommend lowering beyond this number in the absence of a co-morbid condition (e.g., type I diabetes mellitus). This goal also reflects concern that a dBP<80mmHg may limit uteroplacental perfusion270;275.
In contrast, women with co-morbid conditions (Table 1) should probably have their sBP lowered to 130-139mmHg and their dBP lowered to 80-90mmHg. Given that antihypertensive therapy in pregnancy aims to optimize pregnancy outcome (rather than affect long-term cardiovascular risk), other cardiovascular risk markers that are considered ‘compelling indications’ outside of pregnancy are not considered as such in pregnancy: cigarette smoking, abnormal (pre-pregnancy) lipid profile, strong family history of premature cardiovascular disease, truncal obesity or sedentary lifestyle. Choosing a higher BP goal than the non-pregnancy recommendation of BP <130/80mmHg represents a compromise between maternal protection and maintenance of placental perfusion.
For women with pre-eclampsia, data are insufficient to prompt different recommendations for management of a BP of 140-159/90-105mmHg. Antihypertensive therapy does not decrease maternal morbidity in pre-eclampsia or eclampsia, and eclampsia is not simply a hypertensive encephalopathy. However, there may be circumstances (e.g., severe headache) in which it seems prudent to normalize BP, and others in which it may not (e.g., absent end-diastolic flow).
For advice about treatment of secondary causes of hypertension in pregnancy, the reader is referred to the main Canadian Hypertension Society document for initial guidance276.
When a decision is made to use antihypertensive therapy, there is little to guide the choice of agent. In RCTs, a wide variety of antihypertensive agents have been compared with placebo or no therapy: methyldopa, labetalol, other pure beta-blockers (acebutolol, mepindolol, metoprolol, pindolol, and propranolol), calcium channel blockers (isradipine, nicardipine, nifedipine, and verapamil), hydralazine, prazosin, or ketanserin (28 trials, 3,200 women)277; ketanserin, isradipine, nicardipine, and mepindolol are not used in Canada. In comparative trials (usually of beta-blockers compared with methyldopa), beta-blockers (i.e., labetalol, pindolol, metoprolol, or oxprenolol) may be more effective antihypertensives than is methyldopa (RR 0.75, 95% 0.58, 0.94) (10 trials, 539 women), but no other differences in maternal or perinatal outcomes have been demonstrated (19 trials, 1,282 women)180;277;278. Very limited data have not revealed adverse effects of (any) antihypertensive agent on health or neurodevelopment assessed at one year (nifedipine, 110 children)279, 18 months (atenolol, 190 children)280, or 7 ½ years (methyldopa, 242 children)281 in placebo-controlled trials.
Labetalol and methyldopa are the oral agents used most frequently in Canada254 (Table 6). ACE inhibitors and ARBs are fetotoxic282, especially to the fetal kidney; however, an ACE inhibitor or ARB that was prescribed pre-pregnancy for renoprotection should be restarted postpartum, even during breastfeeding283. Thiazide diuretics can be considered for use; despite concerns that thiazides may inhibit the normal plasma volume expansion of pregnancy, thiazides used after the first trimester for pre-eclampsia prevention did not increase adverse maternal or perinatal outcomes but did not prevent pre-eclampsia or severe hypertension (5 trials, 1,836 women)177; there are no follow-up studies on children exposed to thiazides in utero. Specific mention should be made of a few agents. It is not clear why atenolol (in contrast to other even cardioselective beta-blockers) may be associated with adverse effects on fetal growth284-288; until further data are available on the risks of atenolol in pregnancy, other agents are preferable. More stillbirths were reported in the prazosin arm of one trial289. Hydralazine is not recommended because of maternal side effects when used alone290. Oral antihypertensives do not appear to change FHR or pattern, but the quality of the data is poor291; as a conservative approach, changes in FHR or pattern while a woman is taking antihypertensive therapy are best attributed to evolution of the underlying HDP, and not to the antihypertensive agent.
Corticosteroids for acceleration of fetal pulmonary maturity #
Recommendations:
- Antenatal corticosteroid therapy should be considered for all women who present with pre-eclampsia before 34 weeks’ gestation (I, A).
- Antenatal corticosteroid therapy may be considered for women who present at <34 weeks’ with gestational hypertension (despite the absence of proteinuria or ‘adverse conditions’) if delivery is contemplated within the next 7 days (III, I).
Comments:
When administered prior to 34 weeks’, antenatal corticosteroids (i.e., betamethasone 12mg IM every 24hr for two doses) accelerate fetal pulmonary maturity and decrease neonatal mortality and morbidity (www.sogc.org/guidelines, 2003).
If expectantly managed, women with pre-eclampsia will be delivered within two weeks, but the duration of pregnancy prolongation varies from hours to weeks; all such women should receive antenatal corticosteroids for acceleration of fetal pulmonary maturity.
Women with gestational hypertension with neither proteinuria nor ‘adverse conditions’ at <34 weeks’ will evolve into pre-eclampsia in 1/3 of cases; however, delivery takes a mean of about five weeks and is unlikely within the next 7 days292. Whether or not these women should receive antenatal corticosteroids at onset of their gestational hypertension is unclear.
Antenatal corticosteroids may cause significant, transient changes in FHR and variability, up to four days after administration, as measured by either computerized or visual analysis of the CTG293;294 (‘Fetal surveillance’, 2007, www.sogc.org/guidelines).
Mode of delivery #
Recommendations:
- For any HDP , vaginal delivery should be considered unless a Caesarean section is required for the usual obstetric indications (II-2, B).
- If vaginal delivery is planned and the cervix is unfavourable, then cervical ripening should be used to increase the chance of a successful vaginal delivery (I, A).
- Antihypertensive treatment should be continued throughout labour and delivery to maintain sBP at <160mmHg and dBP at <110mmHg (II-2, B)
- The third stage of labour should be actively managed with oxytocin 5 units iv or 10 units IM, particularly in the setting of thrombocytopenia or coagulopathy (I, A).
- Ergometrine should not be used in the management of the third stage of labour in women with HDP (II-3, D).
Comments:
All women with a HDP should be considered for induction of labour.
For induction of labour, cervical ripening is recommended to increase the chance of successful vaginal delivery; these data are derived from normotensive pregnancies (www.sogc.org/guidelines). Induction of labour in severe pre-eclampsia takes more time295 and is less successful296. However, success is 60% at >32 weeks297;298. A success rate of 30% can be achieved even when birthweight is <1500g299;300. The success rate is low (10%) at <26 weeks301. An unfavourable cervix does not preclude successful induction302. Neither intrauterine fetal growth restriction nor oligohydramnios are contraindications to induction of labour303. When there is increased resistance to diastolic flow in the umbilical artery, the vaginal delivery rate is significantly lower but still greater than 50%304;305. Most babies with absent or reversed end-diastolic flow by Doppler velocimetry of the umbilical artery are delivered by Caesarean section306.
With induction of labour, in observational studies, maternal and fetal outcomes are similar or improved in severe pre-eclampsia307-310. There are also future considerations relevant to Caesarean section, such as the risk of uterine rupture with subsequent pregnancies or morbidity associated with repeat Caesarean sections311.
Epidural analgesia lowers BP and possibly, cerebral blood flow index312. Women with pre-eclampsia are at risk of thrombocytopenia and coagulopathy (antepartum or de novo, postpartum), and all standard measures should be taken to avoid postpartum haemorrhage, such as active management of the third stage with oxytocin (www.sogc.guidelines). Ergometrine administration is known to increase blood pressure. While ergometrine is used in some centres in the management of hypotonic postpartum haemorrhage, the use of ergometrine in patients with a HDP should be avoided.
Anaesthesia, including fluid administration #
Recommendations:
- The anesthesiologist should be informed about a woman with pre-eclampsia who is admitted to delivery suite (II-3, B).
- A platelet count should be performed in all women with a HDP on admission to delivery suite, but tests of platelet function are not recommended (III, C).
- Consider regional analgesia/anaesthesia appropriate in women with a platelet count >75×109/L, unless there is a coagulopathy, falling platelets, or co-adminstration of an antiplatelet agent (e.g., ASA) or anticoagulant (e.g., heparin) (III, B).
- Consider regional anaesthesia for women who are taking low-dose ASA in the absence of coagulopathy and in the presence of an adequate platelet count (I, A).
- Consider regional anaesthesia for women on low-molecular weight heparin (LMWH) 12 hours after a prophylactic dose, or 24 hours after a therapeutic dose (III, B).
- Early insertion of an epidural catheter (in the absence of contraindications) is recommended for control of pain (I, A).
- A fixed intravenous fluid bolus should not be administered prior to regional analgesia/anaesthesia (I, D).
- Small doses of phenylephrine or ephedrine may be used to prevent/treat hypotension during regional anaesthesia (I, A).
- In the absence of contraindications , consider all of the following acceptable methods of anaesthesia for women undergoing Caesarean section: epidural, spinal, combined spinal-epidural, and general anaesthesia (I, A).
- Intravenous and oral fluid intake should be minimized in women with pre-eclampsia, to avoid pulmonary edema (II-1, B).
- Fluid administration should not be routinely administered to treat oliguria (<15mL/hr) (III, D).
- For persistent oliguria, neither dopamine nor furosemide is recommended (I, D).
- Central venous access is not routinely recommended, and if inserted, should be used to monitor trends and not absolute values (II-2, D).
- Pulmonary artery catheterization is not recommended unless there is a specific associated indication (III, D), and then in a high dependency unit setting (III, B).
Comments:
It is ideal to effect early consultation with an anesthesiologist, ideally antepartum but certainly on admission to the labour ward in a woman with pre-eclampsia. The importance of communication between care-givers has been repeatedly highlighted by the Confidential Enquiries into Maternal Deaths (UK). The anesthesiologist can assess the patient for specific anaesthetic concerns (including coagulation, airway, previous anaesthetic problems, severity of hypertension, level of consciousness, and medication used such as MgSO4 which interacts with non-depolarizing muscle relaxants). S/he can also facilitate effective management of pre-eclampsia complications (e.g., pulmonary edema), initiate early epidural analgesia, and insert an indwelling arterial catheter in women who need serial blood sampling and/or parenteral antihypertensive medication (and close BP monitoring). Women who are obtunded and/or have evidence of increased intracranial pressure can be administered an appropriate anaesthetic to prevent both an increase in BP with induction and an increase in intracranial pressure.
All women with a HDP should have a platelet count. Tests of platelet function, such as bleeding time, thromboelastrography, or platelet function analyzer 100, are not indicated as there is no evidence that an abnormal result increases bleeding risk313. Both the absolute number of, and trend in, platelet counts are of import. Bleeding into the epidural space following neuroaxial anaesthesia has not been associated with platelet counts above 75×109/L, as long as there is no platelet dysfunction or associated coagulopathy314. Among anesthesiologists, practice varies widely within the range of 50-100×109/L platelets315;316. The same comments made about epidural catheter insertion apply to platelet counts necessary for catheter removal. Women on low-dose ASA are eligible for neuraxial anaesthesia317.
In addition to platelet count, it may be prudent to include tests of coagulation, particularly if there is other end-organ system involvement or platelets are abnormal in number. Regardless, some anesthesiologists will want INR, aPTT and fibrinogen prior to regional analgesia/anaesthesia318;319.
The American Society of Regional Anaesthesia (ASRA) guidelines specify that women are not eligible for regional anaesthesia until at least 10-12 hours after a prophylactic dose, or 24 hours after a therapeutic dose, of LMWH, based on reports of epidural haematoma in non-pregnancy populations320. However, some anesthesiologists prefer to wait 24 hours after any dose of LMWH because of the unknown risk of an epidural haematoma.
Early placement of an epidural catheter is advantageous. First, it maintains the option of regional anaesthesia should the maternal condition subsequently change (e.g., thrombocytopenia develops) or the fetal condition change quickly such that general anaesthesia would otherwise be required. However, should delivery be required in under 5-10minutes, general anaesthesia will still be required, even in the face of an existing epidural catheter. Second, epidural analgesia ablates the labour pain-induced increase in cardiac output and BP mediated by the sympathetic nervous system, which is activated particularly in women with pre-eclampsia321-323. Epidural analgesia does not harm the fetus; in fact, Doppler velocimetry of the umbilical artery may improve324-326. Epidural analgesia does not increase the risk of Caesarean section in women with severe pre-eclampsia327. Combined spinal-epidural anaesthesia is acceptable328.
If there is a contraindication to regional analgesia/anaesthesia, then intravenous opioid analgesia is a reasonable alternative. However, there is a higher risk of neonatal depression and neonates more frequently need naloxone (one small RCT)329.
For Caesarean section in the absence of an epidural catheter, spinal is preferred to epidural as it achieves more rapid and effective anaesthesia and requires use of a smaller needle330. Spinal is preferred to general anaesthesia because spinal avoids the risks of the hypertensive response to intubation; however, spinal anaesthesia may take more time to achieve, and may be associated with lower cord pH and higher cord base deficit, the clinical implications of which are not known331. No differences in uteroplacental hemodynamics have been demonstrated during spinal anaesthesia332.
General anaesthesia in women with a hypertensive disorder is more likely to be associated with difficult (or failed) intubation333;334, and to be associated with a hypertensive response to intubation335;336. This hypertensive response can be attentuated by antihypertensive (such as parenteral labetalol or oral nifedipine), nitroglycerin, or parenteral opioids337-341.
Prior to neuroaxial analgesia/anaesthesia, preloading with a fixed volume of crystalloid (i.e., 500-1,000mL) is neither necessary nor effective in preventing a fall in BP in normal women prior to Caesarean section (meta-analysis of RCTs)342; no studies are available for hypertensive pregnant women. Exceptions to this statement could include dehydration and/or FHR abnormalities. Pre-loading may also increase the risk of pulmonary oedema which is the major cause of death in women with preeclampsia343. If pre-loading is performed, then it may be prudent to use colloid, although concerns have been raised about the potential to cause coagulopathy344.
Oliguria (<15mL urine/hr) is common in the setting of pre-eclampsia, particularly postpartum. In the absence of pre-existing renal disease or a rising creatinine, oliguria should be tolerated, at least over hours. First, oliguria is non-specific and has many causes, including oxytocin administration and high levels of ADH following surgery. Second, pulmonary edema from fluid administration is a leading cause of death in women with pre-eclampsia345, and more fluid administration is associated with more pulmonary edema346;347. Fluid balance should be closely monitored and furosemide should not be administered unless there is pulmonary edema. No conclusions can be drawn about the benefits of furosemide or dopamine for oliguria and these are not recommended348;349.
If hypotension does develop following regional analgesia/anaestheisa, vasopressors should be administered as an infusion or small boluses of ephedrine (5-10mg/bolus) or pheneylephrine (50-100µg/bolus)350. Small doses are recommended to avoid an exaggerated response in hypertensive pregnant women.
Central venous access is recommended only in women who are unstable from a hemodynamic point of view, such as with haemorrhage or acute renal failure. Women can be effectively monitored by vital signs and oxygen saturation. There is no correlation between CVP and pulmonary capillary wedge pressure, so absolute values of CVP are less important than the trend. CVP should be used for monitoring response to therapy, rather than for diagnostics. Pulmonary artery catheterization is not recommended unless there is a specific associated indication and then it should be done in the ICU setting.
Aspects of care specific to women with pre-existing hypertension #
Recommendations:
- Pre-conceptual counselling is recommended (III, I).
- Discontinue ACE inhibitors and ARBs pre-pregnancy (or as soon as pregnancy is diagnosed) (II-2, D).
- If an antihypertensive agent(s) is to be discontinued or changed for the purposes of pregnancy, then consider changing the agent(s) pre-pregnancy if the woman has uncomplicated pre-existing hypertension, or in the presence of co-morbid conditions, she is likely to conceive easily (within 12 months) (III, I).
- Consider discontinuing atenolol when pregnancy is diagnosed (I, D).
- A variety of antihypertensive drugs may be used in the first trimester of pregnancy (e.g., methyldopa, labetalol, and nifedipine) (II-2, B).
Comments:
One percent of women under 30 years of age are hypertensive. Although pre-conceptual counselling is ideal, 50% of pregnancies are unplanned. Therefore, inadvertent exposure to antihypertensives will occur. The adequacy of contraception and the potential for teratogenicity of drugs must be considered when prescribing antihypertensives to women of child-bearing age. All such women should be reminded to take at least 0.8mg/day of folic acid prior to pregnancy.
The potential teratogenicity of antihypertensives must be assessed relative to the baseline risk of major malformations: 1-5% of pregnancies. None of the antihypertensive agents has been proven not to be teratogenic, but the quality of the information is only fair for most agents351. (Information can be obtained rapidly from the DART® database, www.toxnet.nlm.nih.gov). Those antihypertensive agents used most commonly are methyldopa and labetalol. A teratogenic effect of ACE inhibitors has been reported, but the confounding effects of factors related to major malformations (such as pre-gestational diabetes) have not been established352. Angiotensin-II receptor antagonists are considered to have the same potential for teratogenicity, which have been described in a more limited literature353. The potential for atenolol to have adverse effects on fetal growth has been associated in particular, with use from early pregnancy285;287;288;354;355.
Pre-pregnancy or when pregnancy is diagnosed, there is little information to guide whether to replace ACE inhibitors, ARBs, atenolol, or a less commonly used antihypertensive in pregnancy, and if so, with what. There are a number of issues to consider.
- First, what is the indication for the drug? In an otherwise healthy woman with non-severe hypertension, then it is not critical to normalize BP over months. BP falls in pregnancy anyway, reaching a nadir at about 20 weeks’, and then rising towards pre-pregnancy levels by term. It is possible, therefore, that antihypertensive agents may not be needed, or needed in lower dosage towards the end of pregnancy.
- Second, is there an alternative agent available? If, however, ACE inhibitors are being given for renoprotection, no alternative is available. Data are too limited to recommend diltiazem to decrease proteinuria and preserve renal structure and function in pregnant women with chronic renal disease in pregnancy356.
- Third, how long will conception take? It is normal for conception to take up to 12 months, but women over the age of 30years have a higher incidence of subfertility. If an ACE inhibitor is discontinued pre-pregnancy in a woman with renal disease, yet conception does not occur after 12 months and proteinuria is rising despite excellent BP control (i.e., <130/80mmHg), then it may be prudent to reinstate ACE inhibition, perform monthly pregnancy tests, and proceed with investigations of subfertility. The level of proteinuria is a prognostic factor for long-term renal survival.
Women with pre-existing hypertension may have other co-morbidities and/or cardiovascular risk factors that are being treated.
Lovastatin, the statin for which the most information is available in pregnancy, is unlikely to represent a reproductive risk based on published case reports357. However, as the objective of statin therapy is to decrease long-term cardiovascular risk, the potential risks of statin therapy (over the nine months of pregnancy) may outweigh the potential benefits of statin therapy (realized over years of therapy including the nine months of pregnancy). Statin therapy should be discontinued pre-pregnancy or as soon as pregnancy is diagnosed.
Aspirin is recommended for global cardiovascular risk protection in non-dyslipidaemic individuals with hypertension in the presence of three or more major cardiovascular risk markers, including but not limited to: diabetes mellitus, smoking, family history of premature cardiovascular disease, microalbuminuria or proteinuria, total cholesterol to high-density lipoprotein ratio ≥6, or left ventricular hypertrophy358. Low-dose aspirin can be continued throughout pregnancy (See ‘Preventing pre-eclampsia and its complications’).
The reader is referred to other sources for management of renal disease in pregnancy359.
Aspects of care specific to women with pre-eclampsia #
Timing of delivery of pre-eclampsia
Recommendations:
- Patients should be made aware that delivery is the only cure for pre-eclampsia (II-2, A).
- Severe pre-eclampsia warrants obstetric consultation (III, B).
- For women at <34 weeks , expectant management of pre-eclampsia (severe or non-severe) may be considered (I, C). Such care should be provided in a centre capable of supporting a premature neonate (I, C)
- For women at 34-36 weeks with non-severe pre-eclampsia expectant management may be considered (III, I).
- For women at ≥37 weeks with pre-eclampsia (severe or non-severe), delivery should be considered (III, B).
Comments:
The Confidential Enquiries into Maternal Death have consistently identified the failure to appreciate risk in pre-eclampsia as responsible for potentially avoidable complications. Subspecialty consultation has been advised, particularly for women with severe pre-eclampsia360. Given geographical considerations, obstetrical advice could be obtained by telephone.
The phrase, ‘Planned delivery on the best day in the best way’, alludes to the fact that there are a myriad of considerations regarding timing (and mode of) delivery in women with the pre-eclampsia361. When a woman should be delivered will depend on evolving ‘adverse conditions’ (Table 1) and gestational age. As such, the ‘adverse conditions’ in the classification of the HDP do not necessarily represent indications for delivery.

Expectant management of pre-eclampsia refers to attempted pregnancy prolongation following a period of observation, assessment, stabilization (usually of maternal BP), and if gestational age is less than 34 weeks’, administration of corticosteroids for acceleration of fetal pulmonary maturation. Following stabilization, appropriate candidates for expectant management remain undelivered while maternal and fetal well-being are closely monitored. (Details of maternal and fetal surveillance are discussed in the ‘Prognosis’ section.) Expectant management is best considered when the potential perinatal benefits are substantial. As such, the advisability of expectant management is greatly influenced by gestational age, which is the most important determinant of perinatal outcome.
Expectant management of pre-eclampsia at <32-34 weeks may decrease neonatal respiratory distress syndrome, necrotizing enterocolitis, and the need for neonatal intensive care, despite poor fetal growth velocity during the period of pregnancy prolongation (two trials, N=133 women)362;363. The presence and/or magnitude of maternal risk have not been established in adequately powered trials, although rates are very low in uncontrolled observational studies conducted in developed countries364;365. When to delivery these women must be individualized, as there are few published indications for delivery366 that represent absolute indications (e.g, eclampsia), regardless of gestational age.
For women with pre-eclampsia who are late preterm (34-36 weeks) or at term (37-42 weeks), pregnancy prolongation is not expected to have substantial perinatal survival benefits. However, near term, the fetal brain is particularly vulnerable to injury367. Also, delaying delivery may allow time for cervical ripening and successful vaginal delivery. However, there is no literature that evaluates pregnancy prolongation to achieve these goals. In trials comparing one antihypertensive with another near or at term, pregnancy prolongation has been associated with a Caesarean section rate around 70%368-372, with little reported information about other maternal or substantive perinatal outcomes, and no information on the magnitude of pregnancy prolongation.
Magnesium sulphate (MgSO4) for eclampsia prophylaxis or treatment
Recommendations:
- MgSO4 is recommended for first-line treatment of eclampsia (I, A).
- MgSO4 is recommended as prophylaxis against eclampsia in women with severe pre-eclampsia (I, A).
- MgSO4 may be considered for women with non-severe pre-eclampsia (I, C).
- If MgSO4 is either ineffective or there are contraindications to its use , phenytoin or benzodiazepines should not be used as alternatives for eclampsia prophylaxis or treatment.
Comments:
In women with eclampsia, MgSO4 more effectively reduces recurrent seizures than does phenytoin (6 trials, 897 women) or diazepam (7 trials, 1,441 women)373;374. Of note, the protocol for women in the MgSO4 arm of the largest trial, The Eclampsia Trial, did not include administration of benzodiazepines for seizure termination. The initial intravenous treatment protocol was MgSO4 4g iv bolus, followed by an infusion of 1g/hour; a recurrent seizures was treated with another 2-4g iv bolus. Serum Mg levels were not measured, but women were followed clinically for adverse Mg-related effects.
In women with pre-eclampsia [defined in MAGPIE375 as hypertension, ≥1+ proteinuria, and uncertainty about the benefit of MgSO4], MgSO4 (compared with placebo or no therapy in 6 trials, 11,444 women) more than halved the incidence of eclampsia (RR 0.41, 95% CI 0.29 to 0.58)376. The NNT (95% CI) to prevent one seizure among women with severe pre-eclampsia was 50 (34, 100) and for non-severe pre-eclampsia 100 (100, 500). MgSO4 also decreased the risk of abruption [RR 0.64, 95% CI 0.50 to 0.83; NNT of 100 (50, 1000)] but increased the risk of Caesarean section (50% vs. 47%; RR 1.05, 95% CI 1.01 to 1.10). MgSO4 was more frequently associated with side effects (24% vs. 5%; RR 5.26, 95% CI 2.59 to 6.03).
In women with pre-eclampsia, MgSO4 (compared with other agents) also reduced the incidence of eclampsia. MgSO4 (compared with phenytoin in 2 trials, 2241 women)377;378 reduced eclampsia (RR 0.05, 95% CI 0 to 0.84) but increased Caesarean section (RR 1.21, 95% CI 1.05 to 1.41)376. MgSO4 (compared with nimodipine in 1 trial, 1,650 women), reduced eclampsia, but there were more respiratory problems (1.3% vs. 0.4%; RR 3.61, 95% CI 1.01 to 12.91) and the need for additional antihypertensive therapy (54% vs. 46%; RR 1.19, 95% CI 1.08 to 1.31)379. Trials comparing MgSO4 with diazepam (2 trials, 2,241 women) are too small for conclusions to be drawn376.
Therefore, for women with pre-eclampsia, although the risk of eclampsia is lower with MgSO4 (compared with placebo, no therapy or other anticonvulsants), there is ongoing controversy about whether women with non-severe pre-eclampsia benefit overall, particularly as MgSO4 is associated with more Caesarean sections and maternal adverse effects, and is very expensive (i.e., US$23,000 to prevent one seizure if MgSO4 is given to all women with pre-eclampsia)380. In a large American centre that changed its policy from universal prophylaxis of all women with gestational hypertension to a selective approach for only women with severe gestational hypertension, there was more eclampsia and, in those women, more general anaesthesia and adverse neonatal outcomes although absolute rates of these complications were very low381.
Plasma volume expansion for pre-eclampsia
Recommendations:
Plasma volume expansion is not recommended for women with pre-eclampsia (I, E).
Comments:
The rationale for plasma volume expansion for pre-eclampsia is that these women are intravascularly volume contracted and sympathetic tone is high. Colloid has been advocated over crystalloid by some authors, as in healthy women, crystalloid is gone from the intravascular space in 20 minutes382, and possibly sooner in the presence of the endothelial dysfunction of pre-eclampsia. In women with severe pre-eclampsia, observational studies have demonstrated that various types and amounts of crystalloid or colloid have improved maternal haemodynamics383;384, umbilical blood flow velocities385, fetal growth and perinatal mortality384. However, trials (of colloid solution) have demonstrated no improvement in maternal or perinatal outcomes (4 trials, 277 women)386-388 389;390. In a more recent, large trial391, plasma volume expansion was associated with more Caesarean sections, (non-significant) shorter pregnancy prolongation, and a non-significant increase in pulmonary edema. There was also no significant difference in fetal middle cerebral or umbilical artery blood flow velocity, as reported by observational studies.
Therapies for HELLP syndrome specifically
Recommendations:
- Prophylactic transfusion of platelets is not recommended, even prior to Caesarean section, when platelet count is >50×109/L and there is no excessive bleeding or platelet dysfunction (II-2, D).
- Consideration should be given to ordering blood products, including platelets, when platelet count is <50×109/L, platelet count is falling rapidly, and/or there is coagulopathy (III, I).
- Platelet transfusion should be strongly considered prior to vaginal delivery when platelet count is <20×109/L (III, B).
- Platelet transfusion is recommended prior to Caesarean section, when platelet count is <20×109/L (III, B).
- Corticosteriods may be considered for women with a platelet count <50×109/L (III, I).
Comments:
There is general agreement that perioperatively, prophylactic transfusion of platelets is not necessary above 50×109/L392, in the absence of clinical bleeding or platelet dysfunction393. At platelet counts <10-20×109/L, prophylactic transfusion of platelets may be considered as the risk of profound haemorrhage is increased even with non-operative delivery394. In the setting of bleeding, transfusion (of platelets and other blood products) is discussed in the ‘Hemorrhagic Shock’ SOGC guidelines (2002) [www.sogc.org/guidelines].
A D(Rho)-negative woman may develop anti-D antibodies to red blood cells (RBCs) within units of platelets. (Four units of platelets can contain as much as 2mL of RBCs.) In these circumstances, sensitization can be prevented by anti-D prophylaxis, in the form of one 300µg does of anti-D immune globulin; this is sufficient to prevent sensitization following transfusion of up to 30 units of platelets395.
Among women with HELLP (with platelets <50 or <100×109/L), corticosteroids improve maternal haematological and biochemical indices, and possibly the rate of regional anaesthesia396 in observational studies. However, no benefit was demonstrated on important maternal and perinatal outcomes in small, but underpowered, RCTs397.
Women with progressive HELLP syndrome, particularly postpartum, have been described in observational studies to improve with plasma therapies; these are effective for thrombotic thrombocytopenic purpura (TTP) that mimics HELLP398. No RCTs were identified.
Other therapies for treatment of pre-eclampsia
Recommendations:
- Women with pre-eclampsia before 34 weeks’ should receive antenatal corticosteroids for acceleration of fetal pulmonary maturity (I, A).
- Thromboprophylaxis may be considered when bed rest is prescribed (II-2, C).
- Low-dose aspirin is not recommended for treatment of pre-eclampsia (I, E).
- There is insufficient evidence to make recommendations about the usefulness of: activated protein C (III, I); antithrombin (I, I); heparin (III, I); L-arginine (I, I); long-term epidural anaesthesia (I, I); N-acetylcysteine (I, I); probenecid (I, I); or sildenafil nitrate (III, I). Laura, comments in text below suffices
Comments:
There is currently no reliable way of determining which women with pre-eclampsia will develop adverse maternal or fetal conditions that mandate delivery. On average, women with pre-eclampsia remote from term who undergo expectant management of their disease, can have their pregnancies prolonged by two weeks, based on small randomized trials; they should receive antenatal corticosteroids if they present at <34 weeks’399;400.
Pre-eclampsia, many of its risk markers (i.e., obesity, age >35 years, thrombophilia, or renal disease with nephrotic syndrome), and many aspects of its treatment (e.g., bed rest) put women at increased thromboembolic risk. Thromboprophylaxis should be considered in these women antenatally and/or postnatally [as per the relevant SOGC guideline (2000) (www.sogc.org/guidelines)]; although the effectiveness of treatment has not been adequately assessed in pregnancy401, RCTs may not be feasible402.
New therapies for pre-eclampsia are based on its pathogenesis involving vasoconstriction, inflammation, hypercoagulability, and oxidative stress. There is insufficient information to evaluate the effects of:, activated protein C403; antithrombin (3 trials, 185 women)404-406; heparin (no trials)407; long-term epidural anaesthesia (1 trial, 20 women)408; L-arginine (2 rials, 91 women)409;410; N-acetylcysteine (1 trial, 38 women)411; probenecid (1 trial, 40 women)412, or sildenafil nitrate (based on treatment for IUGR413).
Postpartum Treatment #
Postpartum care is divided up into two sections: care in the six weeks postpartum and care six weeks postpartum.
Care in the six weeks postpartum #
Recommendations:
- BP should be measured until day six after delivery (III, B).
- Antihypertensive therapy may be restarted postpartum , particularly in women with severe pre-eclampsia and those who have delivered preterm (II-2, I).
- Severe postpartum hypertension should be treated with antihypertensive therapy, to keep sBP <160mmHg and diastolic BP <110mmHg (II-2, B).
- Antihypertensive therapy may be used to treat non-severe postpartum hypertension , particularly in women with co-morbidities (III, I).
- Antihypertensive agents acceptable for use in breastfeeding include: nifedipine XL®, labetalol, methyldopa, captopril, and enalapril (III, B).
- Should an end-organ dysfunction complicate pre-eclampsia, the patient should be followed up postpartum to assess whether the dysfunction has resolved (III, I).
- Consider withholding non-steroidal anti-inflammatory drugs (NSAIDs) postpartum if hypertension is difficult to control, or there is oliguria, an elevated creatinine (i.e., ≥100µM), or platelets <50×109/L (III, I).
- Postpartum thromboprophylaxis may be considered in women with pre-eclampsia, particularly following antenatal bed rest for more than four days or after Caesarean section (III, I).
- LMWH should not be administered postpartum until at least two hours after epidural catheter removal (III, B).
Comments:
Hypertension may develop for the first time postpartum, with a peak on days 3-6 postpartum due to mobilization of extracellular fluid accumulated during pregnancy. Hypertension may also represent the continuation of an antenatal hypertensive disorder, in up to 50% of women. Women at greatest risk are those with antenatal pre-eclampsia, particularly with preterm delivery, and among multiparous women, those with higher uric acid levels or blood urea nitrogen414;415. In addition to hypertension, the proteinuria and other ‘adverse conditions’ of pre-eclampsia may also worsen postpartum, usually in the first few days, and especially in the setting of severe disease416. Postpartum monitoring is appropriate417, and any end-organ dysfunction should be documented to resolve in the days to weeks after delivery.
There are no reliable data to guide whether or not antenatal antihypertensive therapy should be continued postpartum, or if so, which antihypertensive agent is best418. What is clear is that there is potential for postpartum deterioration in up to 25% of women with pre-eclampsia, so close monitoring is prudent. Regardless, follow-up of BP is warranted.
There is consensus that all severe hypertension should be treated, be it antenatal or postpartum. For non-severe hypertension, the three drug vs. placebo/no treatment trials and three drug vs. drug trials provide insufficient data to guide clinical practice419. Women with co-morbid conditions should be treated according to the CHEP guidelines420. As there are a wide range of agents that are acceptable for use in breastfeeding, clinicians should choose agents with which they are familiar. On average, antihypertensive agents are needed for longer in women with pre-eclampsia (approximately two weeks) compared with those with gestational hypertension without proteinuria (approximately one week), although there is substantial variability between women421. Postpartum follow-up is important, particularly in the week following delivery.
The American Academy of Pediatrics considers the antihypertensives used most commonly in pregnancy to be “usually acceptable” for breastfeeding, in addition to captopril and enalapril422 (Lactomed® database, www.toxnet.nlm.nih.gov). Recommendations are based on an estimated intake by a breastfeeding infant of <10% of a therapeutic dose. However, there are no studies of the effects of antihypertensives on breast-fed preterm infants or those of low birthweight. Also, long-term effects of antihypertensive drug exposure (antenally or through breast milk) have been largely unstudied. Therefore, any adverse effects observed in the infant should be thoroughly evaluated.
NSAIDs, which may exacerbate non-pregnancy hypertension, are self-administered analgesics in many obstetric units and may play a role in contributing to postpartum hypertension, elevated creatinine, or renal failure423.
Pre-eclampsia is a risk marker for postpartum thromboembolism424. Other risk markers are more frequent among these patients, including obesity, bed rest for more than four days prior to delivery, and Caesarean section. Postpartum thromboprophylaxis should be considered (www.sogc.org/guidelines, 2000), although it is of unproven benefit401.
The American Society of Regional Anaestheisa guidelines specify that LMWH should not be administered postpartum (in prophylactic or therapeutic doses) until at least two hours after epidural catheter removal425.
Care beyond six weeks postpartum #
Recommendations:
- Women with a history of severe pre-eclampsia (particularly those who presented or delivered before 34 weeks’) should be screened for pre-existing hypertension (II-2, B), underlying renal disease (II-2 B), and thrombophilia (II-2, C).
- Inter-pregnancy intervals of <2 or ≥10 years are best avoided as both are associated with recurrent pre-eclampsia (II-2, D).
- Women who are overweight should be encouraged to attain a healthy body mass index to decrease risk in future pregnancy (II-2, A) and for long-term health (I, A).
- Women with pre-existing hypertension should undergo the following laboratory investigations (if not done previously): urinalysis; serum sodium, potassium and creatinine; fasting glucose; fasting total cholesterol and high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides; and standard 12-lead electrocardiography (III, I).
- Women who are normotensive but who have had a HDP, may benefit from assessment of traditional cardiovascular risk markers (II-2, B).
- All women who have had a HDP should pursue a healthy diet and lifestyle (I, B) (Table 7).
Comments:
Gestational hypertension usually resolves by six weeks postpartum, but women with severe pre-eclampsia may remain hypertensive (or proteinuric) for up three to six months426.
The recommended investigations or interventions are aimed at either preventing pre-eclampsia or its complications in future pregnancy, or preventing long-term cardiovascular morbidity or mortality.
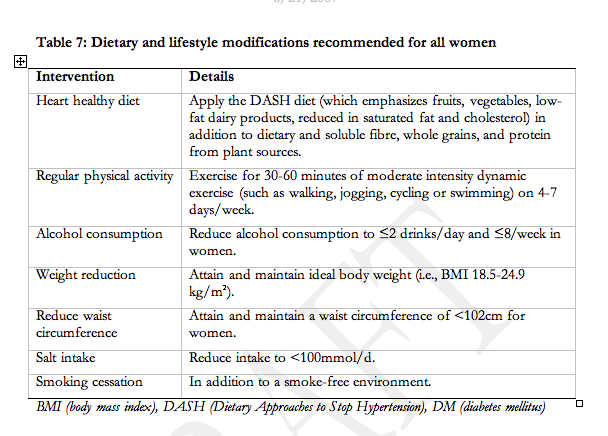
Recommendations regarding future pregnancy #
Thrombophilia appears to confer an increased risk of pre-eclampsia (and other placenta mediated pregnancy complications), but the magnitude of the association appears to be weaker than originally suggested427;428. Also, there is a lack of RCT evidence that permits conclusions about the relative benefits and risks of thromboprophylaxis of thrombophilic women, although it is biologically plausible that such prophylaxis may reduce the incidence of pre-eclampsia in subsequent pregnancies. Thrombophilia testing may, however, influence the choice of contraceptive method.
Screening for other underlying causes of pre-eclampsia (such as renal disease) may better inform management of the woman’s health between or in subsequent pregnancies. Abnormalities detected should prompt referral to the appropriate specialist.
In a prospective study (79 women) with severe obesity, surgical management reduced the risk of gestational hypertension in the subsequent pregnancy429. However, of greater relevance to pregnant women is robust epidemiological data that weight gain between pregnancies (even in non-obese women) is associated with significantly more pre-eclampsia and other pregnancy complications, such as Caesarean section and gestational diabetes198.
Recommendations regarding long-term cardiovascular health #
Women with pre-existing hypertension
Women with pre-existing hypertension should undergo the basic laboratory tests recommended by the CHEP430 (2007 Update, www.hypertension.ca); most should have been performed in pregnancy (and do not need to be repeated), with the exception of fasting lipids and 12-lead EKG. Specific cardiovascular risk factors should be addressed according to existing guidelines. In addition, all women with pre-existing hypertension should comply with CHEP recommendations for dietary and lifestyle modification (Table 7) 431 (2007 Update, www.hypertension.ca).

Women who are normotensive but who had a HDP
Most women who develop a HDP will become normotensive after delivery. However, pregnancy can be regarded as a stress test of sorts, informing women of their future cardiovascular risk432. Large-scale epidemiological studies have associated gestational hypertension, and pre-eclampsia in particular, with an increased risk of hypertension, renal disease433, and cardio- and cerebro-vascular morbidity and mortality434-437. Pre-eclampsia may also be associated with a small increased risk of subsequent thromboembolism438;439. An excess of microalbuminuria has also been documented, but it is unclear whether or not this represents underlying renal disease or an independent cardiovascular risk marker440-442. Whether these effects are genetic and/or influenced by an underlying dysmetabolic syndrome is unclear. Also, whether early testing (and intervention) for traditional cardiovascular risk factors will decrease subsequent vascular events is unproven.
As a routine for all patients, the Canadian Task Force on the Periodic Health Examination [http://www.ctfphc.org] recommends routine cardiovascular risk marker screening only for hypertension and smoking. The Canadian Diabetes Association recommends blood glucose screening at age 40 years (and every 3 years thereafter) [www.diabetes.ca], and the Canadian Working Group on Hypercholesteraemia recommends screening for dyslipidaemia after age 50 years (or menopause) (and every 5 years thereafter)443, assuming that there are no other cardiovascular risk markers.
The CHEP recommends dietary and lifestyle changes (Table 7) for the primary prevention of hypertension. It may be easier to engage women of child-bearing age in these changes following complicated pregnancy. If so, this would be valuable from a public health perspective, given the prevalence and importance of cardiovascular disease in women, and the central role of the woman as care-giver to children, spouses and other family members.
Future Directions #
This represents the second iteration of these guidelines. There are many aspects of diagnosis, evaluation and treatment that must be further clarified. However, some aspects of care are clearly supported by the literature are MgSO4 for severe pre-eclampsia, and antenatal corticosteroids for women with pre-eclampsia before 34 weeks. The following have been identified as priorities: the role of self-measurement of BP, accuracy of the urinary protein:creatinine and albumin:creatinine ratios for diagnosis of proteinuria, multivariable models for prediction of pre-eclampsia, prediction of complications in women with pre-eclampsia, the role of bed rest in the prevention or treatment of pre-eclampsia, the BP goal that optimizes perinatal and maternal outcomes in women with non-severe hypertension, the use of MgSO4 for non-severe pre-eclampsia, and postpartum follow-up and interventions related to future pregnancy and cardiovascular risk. Forthcoming iterations are planned, no less frequently than every three years.
Acknowledgements
This research was supported by the SOGC with additional support from the British Columbia Reproductive Care Programme. The CHS provided assistance with the literature search and travel support for AL.
LM holds a Scholar Award from the Michael Smith Foundation for Health Research (MSFHR). PvD holds a CIHR Scientist Award and a Senior Scholar Award from the MSFHR. SB, XC, JH, SS and FX are supported by scholarships from STIRRHS (Strategic Training Initiative in Research in Reproductive Health Sciences). Much of the Canadian research in this document has been funded by CIHR. The potential for ongoing support of CIHR for research is this field is gratefully acknowledged.
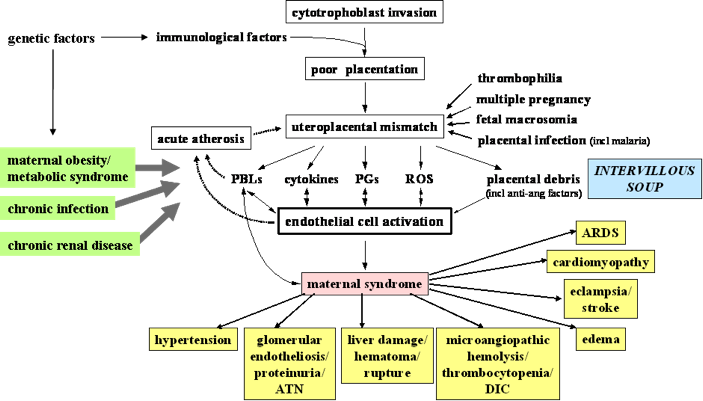

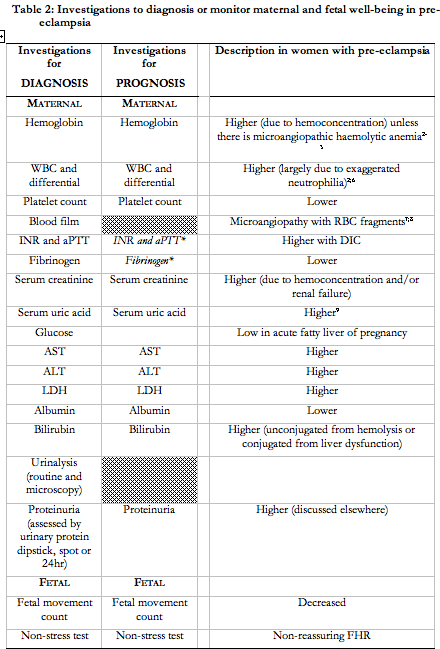
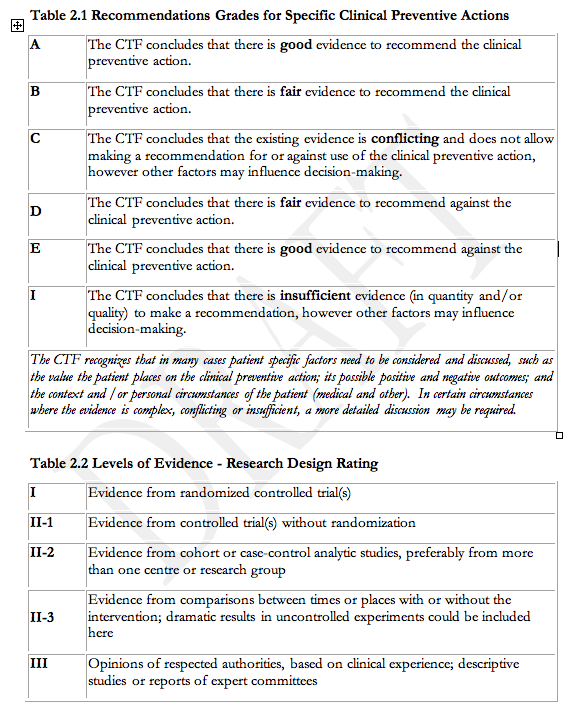
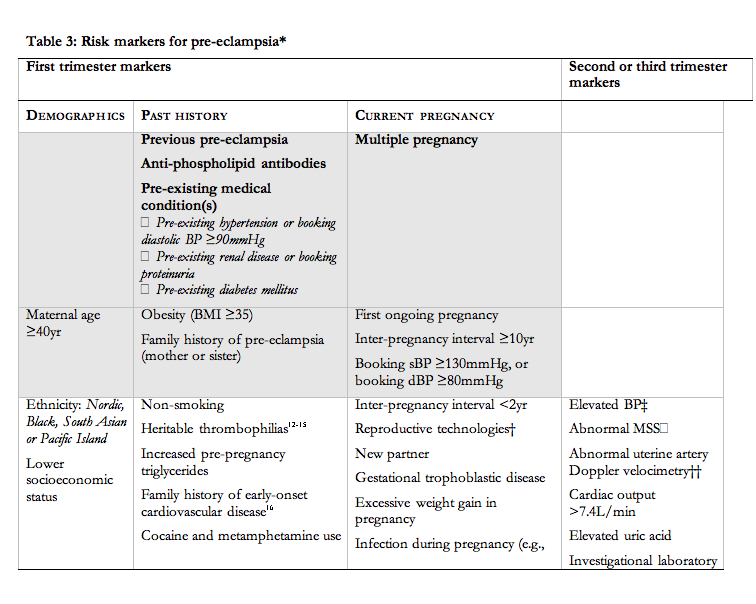
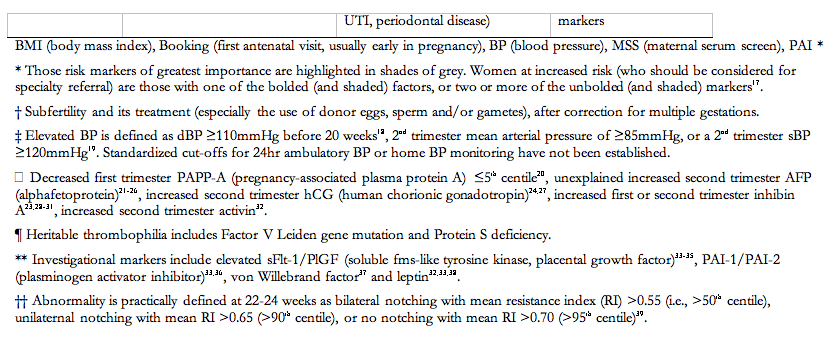

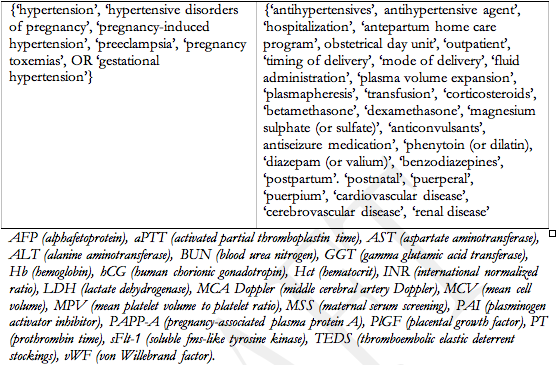
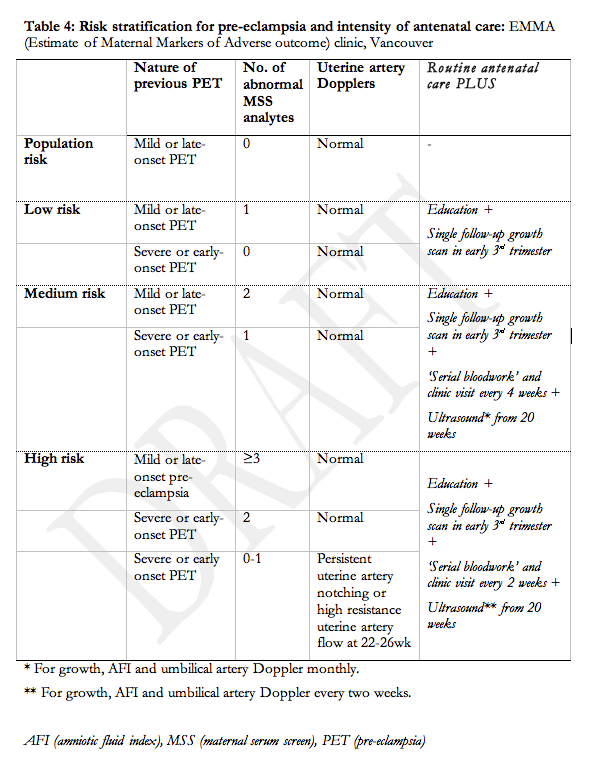
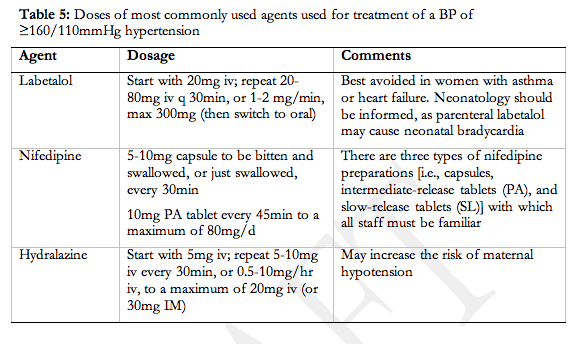
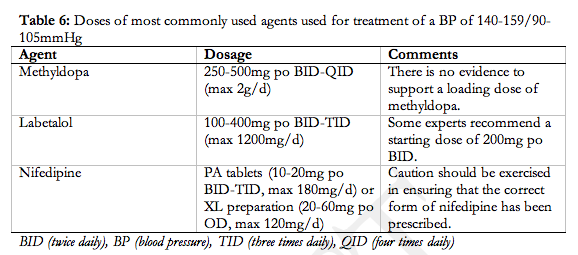
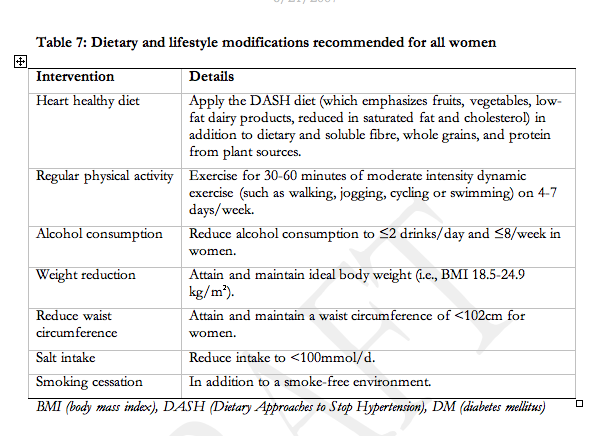
Lectures:
References #
- Health Canada. Special report on maternal mortality and severe morbidity in Canada – Enhanced surveillance: the path to prevention. Ottawa: Minister of Public Works and Government Services Canada, 2004.
- Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy2. Hypertension 2003;41:437-45.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am.J.Obstet.Gynecol. 2000;183:S1-S22.
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust.N.Z.J.Obstet.Gynaecol. 2000;40:133-8.
- Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am.J.Obstet.Gynecol. 2000;183:S1-S22.
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust.N.Z.J.Obstet.Gynaecol. 2000;40:133-8.
- Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 1997;157:715-25.
- Moutquin JM, Garner PR, Burrows RF, Rey E, Helewa ME, Lange IR et al. Report of the Canadian Hypertension Society Consensus Conference: 2. Nonpharmacologic management and prevention of hypertensive disorders in pregnancy. CMAJ. 1997;157:907-19.
- Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of the Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy . CMAJ. 1997;157:1245-54.
- McAlister FA. The Canadian Hypertension Education Program–a unique Canadian initiative . Can.J Cardiol. 2006;22:559-64.
- Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I–Blood pressure measurement, diagnosis and assessment of risk. Can.J Cardiol. 2006;22:573-81.
- Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I–Blood pressure measurement, diagnosis and assessment of risk. Can.J Cardiol. 2006;22:573-81.
- Shennan A, Gupta M, Halligan A, Taylor DJ, de Swiet M. Lack of reproducibility in pregnancy of Korotkoff phase IV as measured by mercury sphygmomanometry . Lancet 1996;347:139-42.
- Brown MA, Buddle ML, Farrell T, Davis G, Jones M. Randomised trial of management of hypertensive pregnancies by Korotkoff phase IV or phase V. Lancet 1998;352:777-81.
- Wichman K, Ryden G, Wichman M. The influence of different positions and Korotkoff sounds on the blood pressure measurements in pregnancy . Acta Obstet Gynecol.Scand Suppl 1984;118:25-8.
- Stryker T, Wilson M, Wilson TW. Accuracy of home blood pressure readings: monitors and operators . Blood Press Monit. 2004;9:143-7.
- Ramsay LE, Williams B, Johnston GD, MacGregor GA, Poston L, Potter JF et al. British Hypertension Society guidelines for hypertension management 1999: summary . BMJ 1999;319:630-5.
- Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension . National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N.Engl.J Med. 1998;339:667-71.
- Ferrazzani S, Caruso A, De Carolis S, Martino IV, Mancuso S. Proteinuria and outcome of 444 pregnancies complicated by hypertension . Am.J.Obstet.Gynecol. 1990;162:366-71.
- Mabie WC, Pernoll ML, Biswas MK. Chronic hypertension in pregnancy . Obstet.Gynecol. 1986;67:197-205.
- Rey E,.Couturier A. The prognosis of pregnancy in women with chronic hypertension . Am.J.Obstet.Gynecol. 1994;171:410-6.
- Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension . Obstet.Gynecol. 1983;61:571-6.
- Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome . Am.J.Obstet.Gynecol. 2001;184:979-83.
- Brown MA,.Buddle ML. The importance of nonproteinuric hypertension in pregnancy . Hypertens Pregn 2002;14:57-65.
- Horsager R, Adams M, Richey S, Leveno KJ, Cunningham FG. Outpatient management of mild pregnancy induced hypertension. 15th Annual Meeting of The Society of Perinatal Obstetricians, Atlanta, Georgia 1995.
- Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study . J Obstet Gynaecol.Can. 2003;25:372-82.
- Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa ME et al. The CHIPS Pilot Trial (Control of Hypertension In Pregnancy Study). BJOG 2007;(in press).
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br.J.Obstet.Gynaecol. 1998;105:1177-84.
- Ramsay LE, Williams B, Johnston GD, MacGregor GA, Poston L, Potter JF et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ 1999;319:630-5.
- Reinders A, Cuckson AC, Lee JT, Shennan AH. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: the Microlife 3BTO-A . BJOG 2005;112:915-20.
- Villar J, Say L, Shennan A, Lindheimer M, Duley L, Conde-Agudelo A et al. Methodological and technical issues related to the diagnosis, screening, prevention, and treatment of pre-eclampsia and eclampsia. Int J Gynaecol.Obstet 2004;85 Suppl 1:S28-S41.
- Stryker T, Wilson M, Wilson TW. Accuracy of home blood pressure readings: monitors and operators . Blood Press Monit. 2004;9:143-7.
- Bellomo G, Narducci PL, Rondoni F, Pastorelli G, Stangoni G, Angeli G et al. Prognostic value of 24-hour blood pressure in pregnancy. JAMA 1999;282:1447-52.
- Brown MA, Mangos G, Davis G, Homer C. The natural history of white coat hypertension during pregnancy . BJOG. 2005;112:601-6.
- Hermida RC,.Ayala DE. Prognostic value of office and ambulatory blood pressure measurements in pregnancy . Hypertension 2002;40:298-303.
- Peek M, Shennan A, Halligan A, Lambert PC, Taylor DJ, de Swiet M. Hypertension in pregnancy: which method of blood pressure measurement is most predictive of outcome? Obstet Gynecol 1996;88:1030-3.
- Penny JA, Halligan AW, Shennan AH, Lambert PC, Jones DR, de Swiet M et al. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: which is the better predictor of severe hypertension? Am.J Obstet Gynecol 1998;178:521-6.
- Bellomo G, Narducci PL, Rondoni F, Pastorelli G, Stangoni G, Angeli G et al. Prognostic value of 24-hour blood pressure in pregnancy . JAMA 1999;282:1447-52.
- Peek M, Shennan A, Halligan A, Lambert PC, Taylor DJ, de Swiet M. Hypertension in pregnancy: which method of blood pressure measurement is most predictive of outcome? Obstet Gynecol 1996;88:1030-3.
- Penny JA, Halligan AW, Shennan AH, Lambert PC, Jones DR, de Swiet M et al. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: which is the better predictor of severe hypertension? Am.J Obstet Gynecol 1998;178:521-6.
- Taylor RS, Freeman L, North RA. Evaluation of ambulatory and self-initiated blood pressure monitors by pregnant and postpartum women. Hypertens Pregnancy 2001;20:25-33.
- Bergel E, Carroli G, Althabe F. Ambulatory versus conventional methods for monitoring blood pressure during pregnancy . Cochrane.Database Syst.Rev. 2002;CD001231.
- Friedman EA,.Neff RK. Pregnancy, outcome as related to hypertension, edema, and proteinuria . Perspect.Nephrol.Hypertens 1976;5:13-22.
- Peek M, Shennan A, Halligan A, Lambert PC, Taylor DJ, de Swiet M. Hypertension in pregnancy: which method of blood pressure measurement is most predictive of outcome? Obstet Gynecol 1996;88:1030-3.
- Brown MA, Mangos G, Davis G, Homer C. The natural history of white coat hypertension during pregnancy . BJOG. 2005;112:601-6.
- Peek M, Shennan A, Halligan A, Lambert PC, Taylor DJ, de Swiet M. Hypertension in pregnancy: which method of blood pressure measurement is most predictive of outcome? Obstet Gynecol 1996;88:1030-3.
- Penny JA, Halligan AW, Shennan AH, Lambert PC, Jones DR, de Swiet M et al. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: which is the better predictor of severe hypertension? Am.J Obstet Gynecol 1998;178:521-6.
- Denolle T, Weber JL, Calvez C, Daniel JC, Cheve MT, Marechaud M et al. Home blood pressure measured telemetrically in hypertensive pregnant women . Am J Hypertens 2002;14:43A.
- Retzke U,.Graf H. [Incidence of hypertension in pregnancy in relation to the definition of hypertension]. Zentralbl.Gynakol. 1994;116:73-5.
- Broughton PF, Sharif J, Lal S. Predicting high blood pressure in pregnancy: a multivariate approach . J Hypertens 1998;16:221-9.
- Why mothers die 1997-1999. The confidential enquiries into maternal deaths in the UK . London: RCOG Press, 2001.
- Martin JN, Jr., Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure . Obstet.Gynecol. 2005;105:246-54.
- Helewa M, Heaman M, Robinson MA, Thompson L. Community-based home-care program for the management of pre-eclampsia: an alternative . CMAJ. 1993;149:829-34.
- Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I–Blood pressure measurement, diagnosis and assessment of risk. Can.J Cardiol. 2006;22:573-81.
- Douglas KA,.Redman CW. Eclampsia in the United Kingdom . BMJ 1994;309:1395-400.
- von Dadelszen P, Magee LA, Devarakonda RM, Hamilton T, Ainsworth LM, Yin R et al. The prediction of adverse maternal outcomes in preeclampsia. J Obstet Gynaecol.Can. 2004;26:871-9.
- Cote AM, Lam E, von Dadelszen P, Magee LA. Accuracy of the 24hr urine collection in hypertensive women. Hypertens Pregnancy 2006;25:230.
- Waugh J, Bell SC, Kilby MD, Lambert P, Shennan A, Halligan A. Urine protein estimation in hypertensive pregnancy: which thresholds and laboratory assay best predict clinical outcome? Hypertens Pregnancy 2005;24:291-302.
- Valerio EG, Ramos JG, Martins-Costa SH, Muller AL. Variation in the urinary protein/creatinine ratio at four different periods of the day in hypertensive pregnant women. Hypertens Pregnancy 2005;24:213-21.
- Al RA, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy . Obstet Gynecol. 2004;104:367-71.
- Durnwald C,.Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia . Am.J Obstet Gynecol. 2003;189:848-52.
- Neithardt AB, Dooley SL, Borensztajn J. Prediction of 24-hour protein excretion in pregnancy with a single voided urine protein-to-creatinine ratio . Am.J Obstet Gynecol. 2002;186:883-6.
- Ramos JG, Martins-Costa SH, Mathias MM, Guerin YL, Barros EG. Urinary protein/creatinine ratio in hypertensive pregnant women . Hypertens Pregnancy 1999;18:209-18.
- Robert M, Sepandj F, Liston RM, Dooley KC. Random protein-creatinine ratio for the quantitation of proteinuria in pregnancy . Obstet Gynecol. 1997;90:893-5.
- Rodriguez-Thompson D,.Lieberman ES. Use of a random urinary protein-to-creatinine ratio for the diagnosis of significant proteinuria during pregnancy . Am.J Obstet Gynecol. 2001;185:808-11.
- Saudan PJ, Brown MA, Farrell T, Shaw L. Improved methods of assessing proteinuria in hypertensive pregnancy . Br.J Obstet Gynaecol. 1997;104:1159-64.
- Yamasmit W, Wongkitisophon K, Charoenvidhya D, Uerpairojkit B, Chaithongwongwatthana S. Correlation between random urinary protein-to-creatinine ratio and quantitation of 24-hour proteinuria in preeclampsia . J Med.Assoc.Thai. 2003;86:69-73.
- Young RA, Buchanan RJ, Kinch RA. Use of the protein/creatinine ratio of a single voided urine specimen in the evaluation of suspected pregnancy-induced hypertension . J Fam.Pract. 1996;42:385-9.
- Zadehmodarres S, Razzaghi MR, Habibi G, Najmi Z, Jam H, Mosaffa N et al. Random urine protein to creatinine ratio as a diagnostic method of significant proteinuria in pre-eclampsia. Aust.N.Z.J Obstet Gynaecol. 2006;46:501-4.
- Cote AM, Brown M, Halstead C, von Dadelszen P, Liston RM, Magee LA. Should the urinary spot protein/creatinine ratio (PCR) be used as a diagnostic test in hypertensive pregnant women: a systematic review. Hypertens Pregnancy 2004;23:36.
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program . Clin Chem. 2006;52:5-18.
- Brown MA,.Buddle ML. Inadequacy of dipstick proteinuria in hypertensive pregnancy . Aust.N.Z.J Obstet Gynaecol. 1995;35:366-9.
- Nisell H, Trygg M, Back R. Urine albumin/creatinine ratio for the assessment of albuminuria in pregnancy hypertension
- Risberg A, Larsson A, Olsson K, Lyrenas S, Sjoquist M. Relationship between urinary albumin and albumin/creatinine ratio during normal pregnancy and pre-eclampsia . Scand J Clin Lab Invest 2004;64:17-23.
- Waugh JJ, Bell SC, Kilby MD, Blackwell CN, Seed P, Shennan AH et al. Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: a study of diagnostic accuracy . BJOG. 2005;112:412-7.
- Wikstrom AK, Wikstrom J, Larsson A, Olovsson M. Random albumin/creatinine ratio for quantification of proteinuria in manifest pre-eclampsia
- Allen VM. The effect of hypertensive disorders in pregnancy on perinatal outcomes: a population-based cohort study. Ottawa: National Library of Canada, 2002.
- Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) . Hypertens Pregnancy 2001;20:IX-XIV.
- Sibai BM. Pitfalls in diagnosis and management of preeclampsia . Am.J Obstet Gynecol. 1988;159:1-5.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am.J.Obstet.Gynecol. 2000;183:S1-S22.
- Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension . National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N.Engl.J Med. 1998;339:667-71.
- Ferrazzani S, Caruso A, De Carolis S, Martino IV, Mancuso S. Proteinuria and outcome of 444 pregnancies complicated by hypertension . Am.J.Obstet.Gynecol. 1990;162:366-71.
- Mabie WC, Pernoll ML, Biswas MK. Chronic hypertension in pregnancy . Obstet.Gynecol. 1986;67:197-205.
- Rey E,.Couturier A. The prognosis of pregnancy in women with chronic hypertension . Am.J.Obstet.Gynecol. 1994;171:410-6.
- Sibai BM, Abdella TN, Anderson GD. Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet.Gynecol. 1983;61:571-6.
- Duckitt K,.Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies . BMJ 2005;330:565.
- Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome . Am.J.Obstet.Gynecol. 2001;184:979-83.
- Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study . J Obstet Gynaecol.Can. 2003;25:372-82.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br.J.Obstet.Gynaecol. 1998;105:1177-84.
- Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – Therapy . Can.J Cardiol. 2006;22:583-93.
- Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001;20:IX-XIV.
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust.N.Z.J.Obstet.Gynaecol. 2000;40:133-8.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am.J.Obstet.Gynecol. 2000;183:S1-S22.
- Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Chan P, Brown M, Simpson JM, Davis G. Proteinuria in pre-eclampsia: how much matters? BJOG. 2005;112:280-5.
- Waugh JJ, Bell SC, Kilby MD, Blackwell CN, Seed P, Shennan AH et al. Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: a study of diagnostic accuracy . BJOG. 2005;112:412-7.
- Fadnes HO, Pape JF, Sundsfjord JA. A study on oedema mechanism in nephrotic syndrome . Scand J Clin Lab Invest 1986;46:533-8.
- Manning RD, Jr.,.Guyton AC. Effects of hypoproteinemia on fluid volumes and arterial pressure
- . Am.J Physiol 1983;245:H284-H293.
- Manning RD, Jr. Effects of hypoproteinemia on renal hemodynamics, arterial pressure, and fluid volume . Am.J Physiol 1987;252:F91-F98.
- Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension . Hypertension 2005;46:1263-9.
- Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review . BJOG. 2006;113:369-78.
- Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome . Am.J.Obstet.Gynecol. 2001;184:979-83.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br.J.Obstet.Gynaecol. 1998;105:1177-84.
- Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study . J Obstet Gynaecol.Can. 2003;25:372-82.
- von Dadelszen P, Magee LA, Lee SK, Stewart SD, Simone C, Koren G et al. Activated protein C in normal human pregnancy and pregnancies complicated by severe preeclampsia: A therapeutic opportunity? Critical Care Medicine 2002;30:1883-92.
- Roberts JM,.Lain KY. Recent Insights into the pathogenesis of pre-eclampsia . Placenta 2002;23:359-72.
- Roberts JM,.Gammill HS. Preeclampsia: recent insights . Hypertension 2005;46:1243-9.
- Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Martin JN, Jr., Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure . Obstet.Gynecol. 2005;105:246-54.
- Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Osmanagaoglu MA, Dinc G, Osmanagaoglu S, Dinc H, Bozkaya H. Comparison of cerebral magnetic resonance and electroencephalogram findings in pre-eclamptic and eclamptic women . Aust.N.Z.J Obstet Gynaecol. 2005;45:384-90.
- Crosby ET,.Preston R. Obstetrical anaesthesia for a parturient with preeclampsia, HELLP syndrome and acute cortical blindness . Can J Anaesth. 1998;45:452-9.
- Demirtas O, Gelal F, Vidinli BD, Demirtas LO, Uluc E, Baloglu A. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn Interv.Radiol. 2005;11:189-94.
- Matsuda H, Sakaguchi K, Shibasaki T, Takahashi H, Kawakami Y, Furuya K et al. Cerebral edema on MRI in severe preeclamptic women developing eclampsia. J Perinat.Med. 2005;33:199-205.
- Na SJ, Hong JM, Park JH, Chung TS, Lee KY. A case of reversible postpartum cytotoxic edema in preeclampsia . J Neurol.Sci. 2004;221:83-7.
- Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy . Radiology 2000;217:371-6.
- Yamaguchi K, Fukuuchi Y, Nogawa S, Dembo T, Tomita Y, Tanaka K. Recovery of decreased local cerebral blood flow detected by the xenon/CT CBF method in a patient with eclampsia . Keio J Med. 2000;49 Suppl 1:A71-A74.
- Redman CWG. The placenta, pre-eclampsia and chronic villitis. In Redman CWG, Sargent IL SP, eds. The Human Placenta, pp 433-67. Oxford: Blackwell Scientific, 1993.
- Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance . Obstet Gynecol.Surv. 2004;59:617-27.
- Xiong X, Demianczuk NN, Saunders LD, Wang FL, Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age . Am.J Epidemiol. 2002;155:203-9.
- Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I–Blood pressure measurement, diagnosis and assessment of risk. Can.J Cardiol. 2006;22:573-81.
- Alkazaleh F, Chaddha V, Viero S, Malik A, Anastasiades C, Sroka H et al. Second-trimester prediction of severe placental complications in women with combined elevations in alpha-fetoprotein and human chorionic gonadotrophin. Am.J Obstet Gynecol. 2006;194:821-7.
- Alkazaleh F, Chaddha V, Viero S, Malik A, Anastasiades C, Sroka H et al. Second-trimester prediction of severe placental complications in women with combined elevations in alpha-fetoprotein and human chorionic gonadotrophin. Am.J Obstet Gynecol. 2006;194:821-7.
- Bailao LA, Osborne NG, Rizzi MC, Bonilla-Musoles F, Duarte G, Bailao TC. Ultrasound markers of fetal infection part 1: viral infections. Ultrasound Q. 2005;21:295-308.
- Baschat AA,.Hecher K. Fetal growth restriction due to placental disease . Semin.Perinatol. 2004;28:67-80.
- Harman CR,.Baschat AA. Comprehensive assessment of fetal wellbeing: which Doppler tests should be performed? Curr.Opin Obstet Gynecol. 2003;15:147-57.
- Duckitt K,.Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies . BMJ 2005;330:565.
- Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community . BMJ 2005;330:576-80.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia . N.Engl.J Med. 2006;355:992-1005.
- Lindheimer MD,.Umans JG. Explaining and predicting preeclampsia. N.Engl.J Med. 2006;355:1056-8.
- Conde-Agudelo A, Villar J, Lindheimer M. World Health Organization systematic review of screening tests for preeclampsia . Obstet Gynecol. 2004;104:1367-91.
- Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326-13.
- von Dadelszen P, Magee LA, Taylor EL, Muir JC, Stewart SD, Sherman P et al. Maternal Hypertension and Neonatal Outcome Among Small for Gestational Age Infants . Obstet Gynecol 2005;106:335-9.
- McCowan LM, Pryor J, Harding JE. Perinatal predictors of neurodevelopmental outcome in small-for-gestational-age children at 18 months of age . Am J Obstet Gynecol 2002;186:1069-75.
- Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community . BMJ 2005;330:576-80.
- Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – Therapy. Can.J Cardiol. 2006;22:583-93.
- Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy . The Jamaica Low Dose Aspirin Study Group. Br.J Obstet Gynaecol. 1998;105:293-9.
- Hauth JC, Goldenberg RL, Parker CR, Jr., Philips JB, III, Copper RL, Dubard MB et al. Low-dose aspirin therapy to prevent preeclampsia . Am.J Obstet Gynecol. 1993;168:1083-91.
- Herabutya Y, Jetsawangsri T, Saropala N. The use of low-dose aspirin to prevent preeclampsia . Int J Gynaecol.Obstet 1996;54:177-8.
- Rotchell YE, Cruickshank JK, Gay MP, Griffiths J, Stewart A, Farrell B et al. Barbados Low Dose Aspirin Study in Pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications . Br.J Obstet Gynaecol. 1998;105:286-92.
- Sibai BM, Caritis SN, Thom E, Klebanoff M, McNellis D, Rocco L et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women . The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N.Engl.J Med. 1993;329:1213-8.
- Hofmeyr GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane .Database Syst.Rev. 2006;3:CD001059.
- Belizan JM,.Villar J. The relationship between calcium intake and edema-, proteinuria-, and hypertension-getosis: an hypothesis . Am.J Clin Nutr. 1980;33:2202-10.
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD et al. Trial of calcium to prevent preeclampsia . N.Engl.J Med. 1997;337:69-76.
- Villar J, Abdel-Aleem H, Merialdi M, Mathai M, Ali MM, Zavaleta N et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women . Am.J Obstet Gynecol. 2006;194:639-49.
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD et al. Trial of calcium to prevent preeclampsia . N.Engl.J Med. 1997;337:69-76.
- Duley L, Henderson-Smart D, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications . Cochrane.Database Syst.Rev. 2005;CD005548.
- Frederick IO, Williams MA, Dashow E, Kestin M, Zhang C, Leisenring WM. Dietary fiber, potassium, magnesium and calcium in relation to the risk of preeclampsia. J Reprod.Med. 2005;50:332-44.
- Kramer MS,.Kakuma R. Energy and protein intake in pregnancy . Cochrane.Database Syst.Rev. 2003;CD000032.
- Rudolf MC,.Sherwin RS. Maternal ketosis and its effects on the fetus . Clin Endocrinol.Metab 1983;12:413-28.
- Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis . J Obstet Gynaecol.Can. 2006;28:680-9.
- Kubik P, Kowalska B, Laskowska-Klita T, Chelchowska M, Leibschang J. [Effect of vitamin-mineral supplementation on the status of some microelements in pregnant women ]. Przegl.Lek. 2004;61:764-8.
- Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. Am.J Epidemiol. 2006;164:470-7.
- Lombardi W, Wilson S, Peniston PB. Wellness intervention with pregnant soldiers . Mil.Med. 1999;164:22-9.
- Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion during prepregnancy physical activity and preeclampsia risk . Med.Sci.Sports Exerc. 2005;37:1836-41.
- Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension . Am J Epidemiol. 2004;160:758-65.
- Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia . Hypertension 2003;41:1273-80.
- Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension . J Epidemiol.Community Health 1989;43:147-52.
- Landsbergis PA,.Hatch MC. Psychosocial work stress and pregnancy-induced hypertension . Epidemiology 1996;7:346-51.
- Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension. J Epidemiol.Community Health 1989;43:147-52.
- Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med.Sci.Sports Exerc. 2006;38:989-1006.
- Kramer MS,.McDonald SW. Aerobic exercise for women during pregnancy . Cochrane.Database Syst.Rev. 2006;3:CD000180.
- Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomized trial. Obstet Gynecol. 2005;106:243-9.
- Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension . J Epidemiol.Community Health 1989;43:147-52.
- Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis . Obstet Gynecol. 2000;95:623-35.
- Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia . Epidemiology 1996;7:245-9.
- Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review . Occup.Environ.Med. 2007;64:228-43.
- Mahomed K. Zinc supplementation in pregnancy. Cochrane .Database Syst.Rev. 2000;CD000230.
- Makrides M,.Crowther CA. Magnesium supplementation in pregnancy. Cochrane .Database Syst.Rev. 2001;CD000937.
- Thaver D, Saeed MA, Bhutta ZA. Pyridoxine (vitamin B6) supplementation in pregnancy. Cochrane.Database Syst.Rev. 2006;CD000179.
- Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction . Cochrane.Database Syst.Rev. 2006;3:CD003402.
- Health Canada: Potential chemical contamination of food. http://www.hc-sc.gc.ca/fn-an/nutrition/prenatal/ . 3-14-2007.Ref Type: Electronic Citation
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy . Cochrane.Database Syst.Rev. 2004;CD001055.
- Coleman T, Thornton J, Britton J, Lewis S, Watts K, Coughtrie MW et al. Protocol for the smoking, nicotine and pregnancy (SNAP) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy . BMC.Health Serv.Res 2007;7:2.
- Churchill D, Beevers G, Meher S, Rhodes C. Diuretics for preventing pre-eclampsia . Cochrane.Database Syst.Rev. 2007;CD004451.
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications . N.Engl.J Med. 2006;354:1796-806.
- Pena-Rosas JP,.Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane.Database Syst.Rev. 2006;3:CD004736.
- Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy . Cochrane.Database Syst.Rev 2001;CD002252.
- Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa ME et al. The CHIPS Pilot Trial (Control of Hypertension In Pregnancy Study) . JOGC 2006;28:416.
- Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa ME et al. The CHIPS Pilot Trial (Control of Hypertension In Pregnancy Study). Hypertens Pregn 2006;25:21.
- Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications . Cochrane.Database Syst.Rev. 2004;CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-8.
- Keim SA,.Klebanoff MA. Aspirin use and miscarriage risk. Epidemiology 2006;17:435-9.
- Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin — a systematic review and meta-analysis of the main randomized controlled trials . Clinics. 2005;60:407-14.
- Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14-16 weeks pregnancy: randomized controlled clinical trial
- Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14-16 weeks pregnancy: randomized controlled clinical trial. Croat.Med.J 2005;46:826-31.
- Caron N, Rivard GE, Rey E. Platelet function analyser (PFA-100) in pregnant women under low dose aspirin (AAS). Thromb Res 2005;115:114.
- Leonhardt A, Bernert S, Watzer B, Schmitz-Ziegler G, Seyberth HW. Low-dose aspirin in pregnancy: maternal and neonatal aspirin concentrations and neonatal prostanoid formation . Pediatrics 2003;111:e77-e81.
- Hermida RC, Ayala DE, Iglesias M. Administration time-dependent influence of aspirin on blood pressure in pregnant women . Hypertension 2003;41:651-6.
- de Swiet M,.Redman CW. Aspirin, extradural anaesthesia and the MRC Collaborative Low-dose Aspirin Study in Pregnancy (CLASP)
- Rudolf MC,.Sherwin RS. Maternal ketosis and its effects on the fetus. Clin Endocrinol .Metab 1983;12:413-28.
- Briley AL, Poston L, Seed PT, Shennan AH. Use of commercially available micronutrient preparations amongst high risk pregnant women taking part in the Vitamins in Pre-eclampsia trial (VIP); relationship to pregnancy outcome. Hypertens Pregn 2006;25:62.
- Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant . Cochrane.Database Syst.Rev. 2005;CD002859.
- Walker MC, Ferguson SE, Allen VM. Heparin for pregnant women with acquired or inherited thrombophilias . Cochrane.Database Syst.Rev. 2003;CD003580.
- Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M et al. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women . Hypertension 2005;45:86-91.
- Villamor E,.Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study . Lancet 2006;368:1164-70.
- Meher S,.Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications . Cochrane.Database Syst.Rev. 2006;CD005942.
- Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med.Sci.Sports Exerc. 2006;38:989-1006.
- Yeo S. A randomized comparative trial of the efficacy and safety of exercise during pregnancy: design and methods. Contemp.Clin Trials 2006;27:531-40.
- Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis . Obstet Gynecol. 2000;95:623-35.
- Meher S,.Duley L. Rest during pregnancy for preventing pre-eclampsia and its complications in women with normal blood pressure. Cochrane.Database Syst.Rev. 2006;CD005939.
- Josten LE, Savik K, Mullett SE, Campbell R, Vincent P. Bedrest compliance for women with pregnancy problems . Birth 1995;22:1-12.
- Han L,.Zhou SM. Selenium supplement in the prevention of pregnancy induced hypertension. Chin Med.J (Engl.) 1994;107:870-1.
- Ziaei S, Hantoshzadeh S, Rezasoltani P, Lamyian M. The effect of garlic tablet on plasma lipids and platelet aggregation in nulliparous pregnants at high risk of preeclampsia. Eur.J Obstet Gynecol.Reprod.Biol. 2001;99:201-6.
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial . Lancet 1999;354:810-6.
- Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial . Am.J Obstet Gynecol. 2005;192:520-1.
- Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens Pregnancy 2006;25:241-53.
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial . Lancet 2006;367:1145-54.
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 2006;367:1145-54.
- Menzies J, Magee LA, MacNab Y, Li J, Yin R, Stuart H et al. Instituting guidelines is associated with a reduced incidence of adverse outcomes in women with pre-eclampsia: a single site study. Obstet.Gynecol. 2007;in press.
- Deruelle P, Coudoux E, Ego A, Houfflin-Debarge V, Codaccioni X, Subtil D. Risk factors for post-partum complications occurring after preeclampsia and HELLP syndrome. A study in 453 consecutive pregnancies. Eur.J Obstet Gynecol.Reprod.Biol. 2006;125:59-65.
- Caetano M, Ornstein MP, von Dadelszen P, Hannah ME, Logan AG, Gruslin A et al. A survey of canadian practitioners regarding diagnosis and evaluation of the hypertensive disorders of pregnancy . Hypertens Pregnancy 2004;23:197-209.
- Chan P, Brown M, Simpson JM, Davis G. Proteinuria in pre-eclampsia: how much matters? BJOG. 2005;112:280-5.
- Ferrazzani S, Caruso A, De Carolis S, Martino IV, Mancuso S. Proteinuria and outcome of 444 pregnancies complicated by hypertension . Am.J Obstet Gynecol. 1990;162:366-71.
- Lao TT, Chin RK, Lam YM. The significance of proteinuria in pre-eclampsia; proteinuria associated with low birth weight only in pre-eclampsia. Eur.J Obstet Gynecol.Reprod.Biol. 1988;29:121-7.
- Newman MG, Robichaux AG, Stedman CM, Jaekle RK, Fontenot MT, Dotson T et al. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria . Am.J Obstet Gynecol. 2003;188:264-8.
- Schiff E, Friedman SA, Kao L, Sibai BM. The importance of urinary protein excretion during conservative management of severe preeclampsia . Am.J Obstet Gynecol. 1996;175:1313-6.
- Chua S,.Redman CW. Prognosis for pre-eclampsia complicated by 5 g or more of proteinuria in 24 hours . Eur.J Obstet Gynecol.Reprod.Biol. 1992;43:9-12.
- Hall DR, Odendaal HJ, Steyn DW, Grove D. Urinary protein excretion and expectant management of early onset, severe pre-eclampsia . Int J Gynaecol.Obstet 2002;77:1-6.
- Newman MG, Robichaux AG, Stedman CM, Jaekle RK, Fontenot MT, Dotson T et al. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria . American Journal of Obstetrics & Gynecology.188(1):264-8, 2003.
- Schiff E, Friedman SA, Kao L, Sibai BM. The importance of urinary protein excretion during conservative management of severe preeclampsia .[see comment]. American Journal of Obstetrics & Gynecology.175(5):1313-6, 1996.
- Neilson JP,.Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane .Database Syst.Rev. 2000;CD000073.
- Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome . Am.J.Obstet.Gynecol. 2001;184:979-83.
- Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study . J Obstet Gynaecol.Can. 2003;25:372-82.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br.J.Obstet.Gynaecol. 1998;105:1177-84.
- Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study . J Obstet Gynaecol.Can. 2003;25:372-82.
- Nabeshima K. [Effect of salt restriction on preeclampsia]. Nippon Jinzo Gakkai Shi 1994;36:227-32.
- HAMLIN RH. The prevention of eclampsia and pre-eclampsia. Lancet 1952;1:64-8.
- Meher S, Abalos E, Carroli G. Bed rest with or without hospitalisation for hypertension during pregnancy . Cochrane.Database Syst.Rev. 2005;CD003514.
- Josten LE, Savik K, Mullett SE, Campbell R, Vincent P. Bedrest compliance for women with pregnancy problems . Birth 1995;22:1-12.
- Greer IA. Epidemiology, risk factors and prophylaxis of venous thrombo-embolism in obstetrics and gynaecology . Baillieres Clin Obstet Gynaecol. 1997;11:403-30.
- Maloni JA, Chance B, Zhang C, Cohen AW, Betts D, Gange SJ. Physical and psychosocial side effects of antepartum hospital bed rest . Nurs.Res 1993;42:197-203.
- Mathews DD, Agarwal V, Shuttleworth TP. A randomized controlled trial of complete bed rest vs ambulation in the management of proteinuric hypertension during pregnancy . Br.J Obstet Gynaecol. 1982;89:131.
- Crowther CA, Bouwmeester AM, Ashurst HM. Does admission to hospital for bed rest prevent disease progression or improve fetal outcome in pregnancy complicated by non-proteinuric hypertension? Br.J Obstet Gynaecol. 1992;99:13-7.
- Leung KY, Sum TK, Tse CY, Law KW, Chan MY. Is in-patient management of diastolic blood pressure between 90 and 100 mm Hg during pregnancy necessary? Hong.Kong.Med.J 1998;4:211-7.
- Mathews DD. A randomized controlled trial of bed rest and sedation or normal activity and non-sedation in the management of non-albuminuric hypertension in late pregnancy . Br.J Obstet Gynaecol. 1977;84:114.
- Rosenberg K,.Twaddle S. Screening and surveillance of pregnancy hypertension–an economic approach to the use of daycare . Baillieres Clin Obstet Gynaecol. 1990;4:89-107.
- Turnbull DA, Wilkinson C, Gerard K, Shanahan M, Ryan P, Griffith EC et al. Clinical, psychosocial, and economic effects of antenatal day care for three medical complications of pregnancy: a randomised controlled trial of 395 women . Lancet 2004;363:1104-9.
- Tuffnell DJ, Lilford RJ, Buchan PC, Prendiville VM, Tuffnell AJ, Holgate MP et al. Randomised controlled trial of day care for hypertension in pregnancy . Lancet 1992;339:224-7.
- Dunlop L, Umstad M, McGrath G, Reidy K, Brennecke S. Cost-effectiveness and patient satisfaction with pregnancy day care for hypertensive disorders of pregnancy . Aust.N.Z.J Obstet Gynaecol. 2003;43:207-12.
- Helewa M, Heaman M, Robinson MA, Thompson L. Community-based home-care program for the management of pre-eclampsia: an alternative. CMAJ . 1993;149:829-34.
- Waugh J, Habiba MA, Bosio P, Boyce T, Shennan A, Halligan AW. Patient initiated home blood pressure recordings are accurate in hypertensive pregnant women . Hypertens Pregnancy 2003;22:93-7.
- Walker S, Permezel M, Brennecke S, Tuttle L, Ugoni A, Higgins J. The effects of hospitalisation on ambulatory blood pressure in pregnancy . Aust.N.Z.J Obstet Gynaecol. 2002;42:493.
- Waugh J, Bosio P, Shennan A, Halligan A. Inpatient monitoring on an outpatient basis: managing hypertensive pregnancies in the community using automated technologies . J Soc.Gynecol.Investig. 2001;8:14-7.
- Barton JR, Istwan NB, Rhea D, Collins A, Stanziano GJ. Cost-savings analysis of an outpatient management program for women with pregnancy-rel
249. Helewa M, Heaman M, Robinson MA, Thompson L. Community-based home-care program for the management of pre-eclampsia: an alternative. CMAJ. 1993;149:829-34.
250. Helewa M, Heaman M, Robinson MA, Thompson L. Community-based home-care program for the management of pre-eclampsia: an alternative. CMAJ. 1993;149:829-34.
251. Barton JR, Stanziano GJ, Sibai BM. Monitored outpatient management of mild gestational hypertension remote from term. Am.J Obstet Gynecol. 1994;170:765-9.
252. Heaman M, Robinson MA, Thompson L, Helewa M. Patient satisfaction with an antepartum home-care program. J Obstet Gynecol.Neonatal Nurs. 1994;23:707-13.
253. Why mothers die 1997-1999. The confidential enquiries into maternal deaths in the UK. London: RCOG Press, 2001.
254. Caetano M, Ornstein M, von Dadelszen P, Hannah ME, Logan AG, Gruslin A et al. A survey of Canadian practitioners regarding the management of the hypertensive disorders of pregnancy. Hypertens Pregn 2003;23:61-74.
255. Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ 2003;327:955-60.
256. Garden A, Davey DA, Dommisse J. Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. Clin.Exp.Hypertens B 1982;1:371-83.
257. Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ 2003;327:955-60.
258. Tuffnell DJ, Jankowicz D, Lindow SW, Lyons G, Mason GC, Russell IF et al. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG. 2005;112:875-80.
259. Scardo JA, Hogg BB, Newman RB. Favorable hemodynamic effects of magnesium sulfate in preeclampsia. Am.J Obstet Gynecol. 1995;173:1249-53.
260. Cotton DB, Gonik B, Dorman KF. Cardiovascular alterations in severe pregnancy-induced hypertension: acute effects of intravenous magnesium sulfate. Am J Obstet Gynecol 1984;148:162-5.
261. Mroczek WJ, Lee WR, Davidov ME. Effect of magnesium sulfate on cardiovascular hemodynamics. Angiology 1977;28:720-4.
262. Pritchard JA. The use of the magnesium ion in the management of eclamptogenic toxemias. Surg.Gynecol.Obstet 1955;100:131-40.
263. Young BK,.Weinstein HM. Effects of magnesium sulfate on toxemic patients in labor. Obstet Gynecol. 1977;49:681-5.
264. Brown MA, Buddle ML, Farrell T, Davis GK. Efficacy and safety of nifedipine tablets for the acute treatment of severe hypertension in pregnancy. Am.J Obstet Gynecol 2002;187:1046-50.
265. Magee LA, Miremadi S, Li J, Cheng C, Ensom MH, Carleton B et al. Therapy with both magnesium sulfate and nifedipine does not increase the risk of serious magnesium-related maternal side effects in women with preeclampsia. Am J Obstet Gynecol 2005;193:153-63.
266. Cetin A, Yurtcu N, Guvenal T, Imir AG, Duran B, Cetin M. The effect of glyceryl trinitrate on hypertension in women with severe preeclampsia, HELLP syndrome, and eclampsia. Hypertens Pregnancy 2004;23:37-46.
267. Neri I, Valensise H, Facchinetti F, Menghini S, Romanini C, Volpe.A. 24-hour ambulatory blood pressure monitoring: a comparison between transdermal glyceryl-trinitrate and oral nifedipine. Hypertens Pregn 1999;18:107-13.
268. Hennessy A, Thornton C, Makris A, Ogle R, Henderson-Smart D, Gillin A et al. Parenteral intravenous optimal therapy trial – a RCT of hydralazine versus mini-bolus diazoxide for hypertensive crises in the obstetric setting. Hypertens Pregn 2006;25:22.
269. Abalos E, Duley L, Steyn D, Henderson-Smart D. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane.Database Syst.Rev. 2007;CD002252.
270. von Dadelszen P,.Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. JOGC 2002;24:941-5.
271. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87-92.
272. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;1:647-9.
273. Reynolds B, Butters L, Evans J, Adams T, Rubin PC. First year of life after the use of atenolol in pregnancy associated hypertension. Arch.Dis.Child 1984;59:1061-3.
274. Khan NA, McAlister FA, Lewanczuk RZ, Touyz RM, Padwal R, Rabkin SW et al. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part II – therapy. Can.J.Cardiol. 2005;21:657-72.
275. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87-92.
276. Khan NA, McAlister FA, Lewanczuk RZ, Touyz RM, Padwal R, Rabkin SW et al. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part II – therapy. Can.J.Cardiol. 2005;21:657-72.
277. Abalos E, Duley L, Steyn D, Henderson-Smart D. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy
1. Cochrane.Database Syst.Rev. 2007;CD002252.
278. Magee LA,.Duley L. Oral beta-blockers for mild to moderate hypertension during pregnancy. Cochrane.Database Syst.Rev 2000;CD002863.
279. Bortolus R, Ricci E, Chatenoud L, Parazzini F. Nifedipine administered in pregnancy: effect on the development of children at 18 months. Br.J.Obstet.Gynaecol. 2000;107:792-4.
280. Reynolds B, Butters L, Evans J, Adams T, Rubin PC. First year of life after the use of atenolol in pregnancy associated hypertension. Arch.Dis.Child 1984;59:1061-3.
281. Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;1:647-9.
282. From the Centers for Disease Control and Prevention. Postmarketing surveillance for angiotensin-converting enzyme inhibitor use during the first trimester of pregnancy–United States, Canada, and Israel, 1987-1995. JAMA 1997;277:1193-4.
283. Beardmore KS, Morris JM, Gallery EDM. Excretion of antihypertensive medication into human breast milk: A systematic review. Hypertension in Pregnancy 2002;21:85-95.
284. Churchill D, Bayliss H, Beevers G. Fetal growth restriction. Lancet 2000;355:1366-7.
285. Easterling T.R., Brateng D., Schmucker B., Brown Z., Millard S.P. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertrension. Obstet.Gynecol. 1999;93:725-33.
286. Easterling TR, Carr DB, Brateng D, Diederichs C, Schmucker B. Treatment of hypertension in pregnancy: effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet Gynecol 2001;98:427-33.
287. Lip G.Y.H., Beevers M., Churchill D., Schaffer L.M., Beevers D.G. Effect of atenolol on birth weight. Am.J.Cardiol. 1997;79:1436-8.
288. Lydakis C., Lip G.Y.H., Beevers M., Beevers D.G. Atenolol and fetal growth in pregnancies complicated by hypertension. Am.J.Hypertens 1999;12:541-7.
289. Hall DR, Odendaal HJ, Steyn DW, Smith M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy; a randomised controlled trial. Br.J.Obstet.Gynaecol. 2000;107:759-65.
290. Rosenfeld JB, Bott-Kanner G, Boner G, Nissenkorn A, Friedman S, Ovadia J et al. Treatment of hypertension during pregnancy with hydralazine monotherapy or with combined therapy with hydralazine and pindolol. Eur.J.Obstet.Gynecol.Reprod.Biol. 1986;22:197-204.
291. Waterman EJ, Magee LA, Lim KI, Skoll A, Rurak D, von Dadelszen P. Do commonly used oral antihypertensives alter fetal or neonatal heart rate characteristics? A systematic review. Hypertens Pregnancy 2004;23:155-69.
292. Magee LA, von Dadelszen P, Bohun CM, Rey E, El Zibdeh M, Stalker S et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study. J Obstet Gynaecol.Can. 2003;25:372-82.
293. Rotmensch S, Lev S, Kovo M, Efrat Z, Zahavi Z, Lev N et al. Effect of betamethasone administration on fetal heart rate tracing: a blinded longitudinal study. Fetal Diagn.Ther. 2005;20:371-6.
294. Subtil D, Tiberghien P, Devos P, Therby D, Leclerc G, Vaast P et al. Immediate and delayed effects of antenatal corticosteroids on fetal heart rate: a randomized trial that compares betamethasone acetate and phosphate, betamethasone phosphate, and dexamethasone. Am.J Obstet Gynecol. 2003;188:524-31.
295. Griffiths AN, Hikary N, Sizer AR. Induction to delivery time interval in patients with and without preeclampsia: a retrospective analysis. Acta Obstet Gynecol.Scand 2002;81:867-9.
296. Xenakis EM, Piper JM, Field N, Conway D, Langer O. Preeclampsia: is induction of labor more successful? Obstet Gynecol. 1997;89:600-3.
297. Blackwell SC, Redman ME, Tomlinson M, Landwehr JB, Jr., Tuynman M, Gonik B et al. Labor induction for the preterm severe pre-eclamptic patient: is it worth the effort? J Matern.Fetal Med. 2001;10:305-11.
298. Nassar AH, Adra AM, Chakhtoura N, Gomez-Marin O, Beydoun S. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am.J Obstet Gynecol. 1998;179:1210-3.
299. Alexander JM, Bloom SL, McIntire DD, Leveno KJ. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-8.
300. Regenstein AC, Laros RK, Jr., Wakeley A, Kitterman JA, Tooley WH. Mode of delivery in pregnancies complicated by preeclampsia with very low birth weight infants. J Perinatol. 1995;15:2-6.
301. Blackwell SC, Redman ME, Tomlinson M, Landwehr JB, Jr., Tuynman M, Gonik B et al. Labor induction for the preterm severe pre-eclamptic patient: is it worth the effort? J Matern.Fetal Med. 2001;10:305-11.
302. Regenstein AC, Laros RK, Jr., Wakeley A, Kitterman JA, Tooley WH. Mode of delivery in pregnancies complicated by preeclampsia with very low birth weight infants. J Perinatol. 1995;15:2-6.
303. Alexander JM, Bloom SL, McIntire DD, Leveno KJ. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-8.
304. Li H, Gudmundsson S, Olofsson P. Prospect for vaginal delivery of growth restricted fetuses with abnormal umbilical artery blood flow. Acta Obstet Gynecol.Scand 2003;82:828-33.
305. Skinner J, Greene RA, Gardeil F, Stuart B, Turner MJ. Does increased resistance on umbilical artery Doppler preclude a trial of labour? Eur.J Obstet Gynecol.Reprod.Biol. 1998;79:35-8.
306. Weiss E, Ulrich S, Berle P. Condition at birth of infants with previously absent or reverse umbilical artery end-diastolic flow velocities. Arch.Gynecol.Obstet 1992;252:37-43.
307. Alexander JM, Bloom SL, McIntire DD, Leveno KJ. Severe preeclampsia and the very low birth weight infant: is induction of labor harmful? Obstet Gynecol. 1999;93:485-8.
308. Blackwell SC, Redman ME, Tomlinson M, Landwehr JB, Jr., Tuynman M, Gonik B et al. Labor induction for the preterm severe pre-eclamptic patient: is it worth the effort? J Matern.Fetal Med. 2001;10:305-11.
309. Coppage KH,.Polzin WJ. Severe preeclampsia and delivery outcomes: is immediate cesarean delivery beneficial? Am.J Obstet Gynecol. 2002;186:921-3.
310. Nassar AH, Adra AM, Chakhtoura N, Gomez-Marin O, Beydoun S. Severe preeclampsia remote from term: labor induction or elective cesarean delivery? Am.J Obstet Gynecol. 1998;179:1210-3.
311. Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176:455-60.
312. Williams KP,.Wilson S. Evaluation of cerebral perfusion pressure changes in laboring women: effects of epidural anesthesia. Ultrasound Obstet Gynecol. 1999;14:393-6.
313. Samama CM. Should a normal thromboelastogram allow us to perform a neuraxial block? A strong word of warning. Can.J Anaesth. 2003;50:761-3.
314. Vigil-De Gracia P, Silva S, Montufar C, Carrol I, De Los RS. Anesthesia in pregnant women with HELLP syndrome. Int J Gynaecol.Obstet 2001;74:23-7.
315. Beilin Y, Bodian CA, Haddad EM, Leibowitz AB. Practice patterns of anesthesiologists regarding situations in obstetric anesthesia where clinical management is controversial. Anesth.Analg. 1996;83:735-41.
316. Wee L, Sinha P, Lewis M. Central nerve block and coagulation: a survey of obstetric anaesthetists. Int J Obstet Anesth. 2002;11:170-5.
317. de Swiet M,.Redman CW. Aspirin, extradural anaesthesia and the MRC Collaborative Low-dose Aspirin Study in Pregnancy (CLASP). Br.J Anaesth. 1992;69:109-10.
318. Barker P,.Callander CC. Coagulation screening before epidural analgesia in pre-eclampsia. Anaesthesia 1991;46:64-7.
319. Beilin Y, Bodian CA, Haddad EM, Leibowitz AB. Practice patterns of anesthesiologists regarding situations in obstetric anesthesia where clinical management is controversial. Anesth.Analg. 1996;83:735-41.
320. Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth.Pain Med. 2003;28:172-97.
321. Moore TR, Key TC, Reisner LS, Resnik R. Evaluation of the use of continuous lumbar epidural anesthesia for hypertensive pregnant women in labor. Am.J Obstet Gynecol. 1985;152:404-12.
322. Newsome LR, Bramwell RS, Curling PE. Severe preeclampsia: hemodynamic effects of lumbar epidural anesthesia. Anesth.Analg. 1986;65:31-6.
323. Shnider SM, Abboud TK, Artal R, Henriksen EH, Stefani SJ, Levinson G. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am.J Obstet Gynecol. 1983;147:13-5.
324. Jouppila P, Jouppila R, Hollmen A, Koivula A. Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol. 1982;59:158-61.
325. Moore TR, Key TC, Reisner LS, Resnik R. Evaluation of the use of continuous lumbar epidural anesthesia for hypertensive pregnant women in labor. Am.J Obstet Gynecol. 1985;152:404-12.
326. Ramos-Santos E, Devoe LD, Wakefield ML, Sherline DM, Metheny WP. The effects of epidural anesthesia on the Doppler velocimetry of umbilical and uterine arteries in normal and hypertensive patients during active term labor. Obstet Gynecol. 1991;77:20-6.
327. Hogg B, Hauth JC, Caritis SN, Sibai BM, Lindheimer M, Van Dorsten JP et al. Safety of labor epidural anesthesia for women with severe hypertensive disease. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am.J Obstet Gynecol. 1999;181:1096-101.
328. Ramanathan J, Vaddadi AK, Arheart KL. Combined spinal and epidural anesthesia with low doses of intrathecal bupivacaine in women with severe preeclampsia: a preliminary report. Reg Anesth.Pain Med. 2001;26:46-51.
329. Head BB, Owen J, Vincent RD, Jr., Shih G, Chestnut DH, Hauth JC. A randomized trial of intrapartum analgesia in women with severe preeclampsia. Obstet Gynecol. 2002;99:452-7.
330. Wee L, Sinha P, Lewis M. Central nerve block and coagulation: a survey of obstetric anaesthetists. Int J Obstet Anesth. 2002;11:170-5.
331. Dyer RA, Farina Z, Joubert IA, Du TP, Meyer M, Torr G et al. Crystalloid preload versus rapid crystalloid administration after induction of spinal anaesthesia (coload) for elective caesarean section. Anaesth.Intensive Care 2004;32:351-7.
332. Karinen J, Rasanen J, Alahuhta S, Jouppila R, Jouppila P. Maternal and uteroplacental haemodynamic state in pre-eclamptic patients during spinal anaesthesia for Caesarean section. Br.J Anaesth. 1996;76:616-20.
333. Brimacombe J. Acute pharyngolaryngeal oedema and pre-eclamptic toxaemia. Anaesth.Intensive Care 1992;20:97-8.
334. Rocke DA,.Scoones GP. Rapidly progressive laryngeal oedema associated with pregnancy-aggravated hypertension. Anaesthesia 1992;47:141-3.
335. Connell H, Dalgleish JG, Downing JW. General anaesthesia in mothers with severe pre-eclampsia/eclampsia. Br.J Anaesth. 1987;59:1375-80.
336. Ramanathan J, Coleman P, Sibai B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth.Analg. 1991;73:772-9.
337. Hood DD, Dewan DM, James FM, III, Floyd HM, Bogard TD. The use of nitroglycerin in preventing the hypertensive response to tracheal intubation in severe preeclampsia. Anesthesiology 1985;63:329-32.
338. Kumar N, Batra YK, Bala I, Gopalan S. Nifedipine attenuates the hypertensive response to tracheal intubation in pregnancy-induced hypertension. Can.J Anaesth. 1993;40:329-33.
339. Ramanathan J, Sibai BM, Mabie WC, Chauhan D, Ruiz AG. The use of labetalol for attenuation of the hypertensive response to endotracheal intubation in preeclampsia. Am.J Obstet Gynecol. 1988;159:650-4.
340. Ramanathan J, Coleman P, Sibai B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth.Analg. 1991;73:772-9.
341. Rout CC,.Rocke DA. Effects of alfentanil and fentanyl on induction of anaesthesia in patients with severe pregnancy-induced hypertension. Br.J Anaesth. 1990;65:468-74.
342. Morgan PJ. The effect of increasing central blood volume to decrease the incidence of hypotension following spinal anesthesia for cesarean section. In Halpern SH, Douglas MJ, eds. Evidence-based Obstetric Anesthesia, pp 89-100. Massachusetts: BMJ Books, Blackwell Publishing, 2005.
343. Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
344. Ngan Kee WD, Khaw KS, Lee BB, Ng FF, Wong MM. Randomized controlled study of colloid preload before spinal anaesthesia for caesarean section. Br.J Anaesth. 2001;87:772-4.
345. Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
346. Ganzevoort W, Rep A, Bonsel GJ, De Vries JI, Wolf H. A randomized trial of plasma volume expansion in hypertensive disorders of pregnancy: influence on the pulsatility indices of the fetal umbilical artery and middle cerebral artery. Am.J Obstet Gynecol. 2005;192:233-9.
347. Thornton C, Hennessy A, vDP, Nishi C, Makris A, Ogle R. International benchmarking – It can be achieved! Hypertens Pregn 2006;25:139.
348. Keiseb J, Moodley J, Connolly CA. Comparison of the efficacy of continuous furosemide and low-dose dopamine infusion in preeclampsia/eclampsia-related oliguria in the immediate postpartum period. Hypertens.Pregnancy. 2002;21:225-34.
349. Nasu K, Yoshimatsu J, Anai T, Miyakawa I. Low-dose dopamine in treating acute renal failure caused by preeclampsia. Gynecol.Obstet.Invest 1996;42:140-1.
350. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth.Analg. 2002;94:920-6, table.
351. Friedman JM. ACE inhibitors and congenital anomalies. N.Engl.J Med. 2006;354:2498-500.
352. Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N.Engl.J Med. 2006;354:2443-51.
353. Losartan. Micromedex Healthcare Series (TERIS) 127. 12-1-2003.
Ref Type: Electronic Citation
354. Churchill D, Bayliss H, Beevers G. Fetal growth restriction. Lancet 2000;355:1366-7.
355. Easterling TR, Carr DB, Brateng D, Diederichs C, Schmucker B. Treatment of hypertension in pregnancy: effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet Gynecol 2001;98:427-33.
356. Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J.Matern.Fetal Neonatal Med. 2002;12:408-12.
357. Lovastatin. TERIS . 2006. Thomson Healthcare Inc.
Ref Type: Electronic Citation
358. Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – Therapy. Can.J Cardiol. 2006;22:583-93.
359. Davison JM. Renal disorders in pregnancy. Curr.Opin Obstet Gynecol. 2001;13:109-14.
360. Why mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
361. Tuffnell DJ, Jankowicz D, Lindow SW, Lyons G, Mason GC, Russell IF et al. Outcomes of severe pre-eclampsia/eclampsia in Yorkshire 1999/2003. BJOG. 2005;112:875-80.
362. Odendaal HJ, Pattinson RC, Bam R, Grove D, Kotze TJ. Aggressive or expectant management for patients with severe preeclampsia between 28-34 weeks’ gestation: a randomized controlled trial. Obstet Gynecol 1990;76:1070-5.
363. Sibai BM, Mercer BM, Schiff E, Friedman SA. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomized controlled trial. Am J Obstet Gynecol 1994;171:818-22.
364. Magee LA, Cote AM, Yong P, Chen I, Gray C, von Dadelszen P. Quantifying the perinatal risks associated with expectant management of pre-eclampsia remote from term: a structured review. Hypertens Pregn 2006;25:140.
365. Magee LA, Cote AM, Yong P, Chen I, Gray C, von Dadelszen P. Quantifying the maternal risks associated with expectant management of pre-eclampsia remote from term: a structured review. Hypertens Pregn 2006;25:201.
366. Schiff E, Friedman SA, Sibai BM. Conservative management of severe preeclampsia remote from term. Obstet Gynecol 1994;84:626-30.
367. Adams-Chapman I. Neurodevelopmental outcome of the late preterm infant. Clin Perinatol. 2006;33:947-64.
368. Gul A, Aslan H, Cebeci A, Polat I, Ulusoy S, Ceylan Y. Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004;26:557-62.
369. Sagen N, Haram K, Nilsen ST. Serum urate as a predictor of fetal outcome in severe pre-eclampsia. Acta Obstetricia et Gynecologica Scandinavica.63(1):71-5, 1984.
370. Varma TR. Serum uric acid levels as an index of fetal prognosis in pregnancies complicated by pre-existing hypertension and pre-eclampsia of pregnancy. International Journal of Gynaecology & Obstetrics. 1920;401-8.
371. Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaptation in hypertensive pregnancy–a study of labetalol vs hydralazine treatment. J Perinat.Med. 1993;21:69-75.
372. Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam S. Randomised controlled trial of methyldopa and isradipine in preeclampsia–effects on uteroplacental and fetal hemodynamics. J Perinat.Med. 1996;24:177-84.
373. Duley L,.Henderson-Smart D. Magnesium sulphate versus diazepam for eclampsia. Cochrane.Database.Syst.Rev. 2000;CD000127.
374. Duley L,.Henderson-Smart D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane.Database.Syst.Rev. 2003;CD000128.
375. Duley L, Farrell B, Spark P, Roberts B, Watkins K, Bricker L et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002;359:1877-90.
376. Duley L, Gulmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane.Database.Syst.Rev. 2003;CD000025.
377. Friedman MN. Magnesium sulfate versus phenytoin for the prevention of eclampsia. N.Engl.J Med. 1995;333:1638.
378. Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N.Engl.J Med. 1995;333:201-5.
379. Belfort MA, Anthony J, Saade GR, Allen JC, Jr. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N.Engl.J Med. 2003;348:304-11.
380. Simon J, Gray A, Duley L. Cost-effectiveness of prophylactic magnesium sulphate for 9996 women with pre-eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG 2006;113:144-51.
381. Alexander JM, McIntire DD, Leveno KJ, Cunningham FG. Selective magnesium sulfate prophylaxis for the prevention of eclampsia in women with gestational hypertension. Obstet.Gynecol 2006;108:826-32.
382. Ueyama H, He YL, Tanigami H, Mashimo T, Yoshiya I. Effects of crystalloid and colloid preload on blood volume in the parturient undergoing spinal anesthesia for elective Cesarean section. Anesthesiology 1999;91:1571-6.
383. Cloeren SE,.Lippert TH. Effect of plasma expanders in toxemia of pregnancy. N.Engl.J Med. 1972;287:1356-7.
384. Visser W, van Pampus MG, Treffers PE, Wallenburg HC. Perinatal results of hemodynamic and conservative temporizing treatment in severe pre-eclampsia. Eur.J Obstet Gynecol.Reprod.Biol. 1994;53:175-81.
385. Karsdorp VH, van Vugt JM, Dekker GA, van Geijn HP. Reappearance of end-diastolic velocities in the umbilical artery following maternal volume expansion: a preliminary study. Obstet Gynecol. 1992;80:679-83.
386. Duley L, Williams J, Henderson-Smart DJ. Plasma volume expansion for treatment of women with pre-eclampsia. Cochrane.Database Syst.Rev. 2000;CD001805.
387. Lowe SA, Hetmanski SJ, Macdonald I, Pipkin F, Rubin PC. Intravenous volume expansion therapy in pregnancy-induced hypertension: the role of vasoactive hormones. Hypertens Pregn 1993;12:139-51.
388. Sehgal NN,.Hitt JR. Plasma volume expansion in the treatment of pre-eclampsia. Am.J Obstet Gynecol. 1980;138:165-8.
389. Belfort M, Uys P, Dommisse J, Davey DA. Haemodynamic changes in gestational proteinuric hypertension: the effects of rapid volume expansion and vasodilator therapy. Br.J Obstet Gynaecol. 1989;96:634-41.
390. Ganzevoort W, Rep A, Bonsel GJ, Fetter WP, van Sonderen L, De Vries JI et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG. 2005;112:1358-68.
391. Ganzevoort W, Rep A, Bonsel GJ, Fetter WP, van Sonderen L, De Vries JI et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG. 2005;112:1358-68.
392. Rebulla P. Platelet transfusion trigger in difficult patients. Transfus.Clin.Biol. 2001;8:249-54.
393. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006;105:198-208.
394. ACOG technical bulletin. Blood component therapy. Number 199–November 1994 (replaces no. 78, July 1984). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol.Obstet 1995;48:233-8.
395. ACOG technical bulletin. Blood component therapy. Number 199–November 1994 (replaces no. 78, July 1984). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol.Obstet 1995;48:233-8.
396. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit of corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: impact on the rate of regional anesthesia. Am.J Obstet Gynecol. 2002;186:475-9.
397. Matchaba P,.Moodley J. Corticosteroids for HELLP syndrome in pregnancy. Cochrane.Database.Syst.Rev. 2004;CD002076.
398. Nguyen TC, Stegmayr B, Busund R, Bunchman TE, Carcillo JA. Plasma therapies in thrombotic syndromes. Int J Artif.Organs 2005;28:459-65.
399. Odendaal HJ, Pattinson RC, Bam R, Grove D, Kotze TJ. Aggressive or expectant management for patients with severe preeclampsia between 28-34 weeks’ gestation: a randomized controlled trial. Obstet Gynecol 1990;76:1070-5.
400. Sibai BM, Mercer BM, Schiff E, Friedman SA. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomized controlled trial. Am J Obstet Gynecol 1994;171:818-22.
401. Gates S, Brocklehurst P, Davis LJ. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane.Database.Syst.Rev. 2002;CD001689.
402. Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low-molecular-weight heparin. Am.J Obstet Gynecol. 2004;191:1296-303.
403. von Dadelszen P, Magee LA, Lee SK, Stewart SD, Simone C, Koren G et al. Activated protein C in normal human pregnancy and pregnancies complicated by severe preeclampsia: A therapeutic opportunity? Critical Care Medicine 2002;30:1883-92.
404. Kobayashi T, Terao T, Ikenoue T, Sameshima H, Nakabayashi M, Kajiwara Y et al. Treatment of severe preeclampsia with antithrombin concentrate: results of a prospective feasibility study. Semin.Thromb.Hemost. 2003;29:645-52.
405. Paternoster DM, Fantinato S, Manganelli F, Milani M, Nicolini U, Girolami A. Efficacy of AT in pre-eclampsia: a case-control prospective trial. Thromb.Haemost. 2004;91:283-9.
406. Maki M, Kobayashi T, Terao T, Ikenoue T, Satoh K, Nakabayashi M et al. Antithrombin therapy for severe preeclampsia: results of a double-blind, randomized, placebo-controlled trial. BI51.017 Study Group. Thromb.Haemost. 2000;84:583-90.
407. Howie PW, Prentice CR, Forbes CD. Failure of heparin therapy to affect the clinical course of severe pre-eclampsia. Br.J Obstet Gynaecol. 1975;82:711-7.
408. Kanayama N, Belayet HM, Khatun S, Tokunaga N, Sugimura M, Kobayashi T et al. A new treatment of severe pre-eclampsia by long-term epidural anaesthesia. J Hum.Hypertens 1999;13:167-71.
409. Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur.J Clin Invest 2005;35:32-7.
410. Staff AC, Berge L, Haugen G, Lorentzen B, Mikkelsen B, Henriksen T. Dietary supplementation with L-arginine or placebo in women with pre-eclampsia. Acta Obstet Gynecol.Scand 2004;83:103-7.
411. Roes EM, Raijmakers MT, Boo TM, Zusterzeel PL, Merkus HM, Peters WH et al. Oral N-acetylcysteine administration does not stabilise the process of established severe preeclampsia. Eur.J Obstet Gynecol.Reprod.Biol. 2006;127:61-7.
412. Schackis RC. Hyperuricaemia and preeclampsia: is there a pathogenic link? Med.Hypotheses 2004;63:239-44.
413. Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol.Metab 2005;90:2550-5.
414. Ferrazzani S, De Carolis S, Pomini F, Testa AC, Mastromarino C, Caruso A. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am.J.Obstet.Gynecol. 1994;171:506-12.
415. Tan LK,.de Swiet M. The management of postpartum hypertension. Bjog-An International Journal of Obstetrics and Gynaecology 2002;109:733-6.
416. Deruelle P, Coudoux E, Ego A, Houfflin-Debarge V, Codaccioni X, Subtil D. Risk factors for post-partum complications occurring after preeclampsia and HELLP syndrome. A study in 453 consecutive pregnancies. Eur.J Obstet Gynecol.Reprod.Biol. 2006;125:59-65.
417. Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am.J Obstet Gynecol. 2004;190:1464-6.
418. Sadeghi S,.Magee LA. Treatment for postpartum hypertension (Protocol for a Cochrane Review). The Cochrane Library 2003.
419. Magee L,.Sadeghi S. Prevention and treatment of postpartum hypertension. Cochrane.Database.Syst.Rev. 2005;CD004351.
420. Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – Therapy. Can.J Cardiol. 2006;22:583-93.
421. Ferrazzani S, De Carolis S, Pomini F, Testa AC, Mastromarino C, Caruso A. The duration of hypertension in the puerperium of preeclamptic women: relationship with renal impairment and week of delivery. Am.J.Obstet.Gynecol. 1994;171:506-12.
422. Transfer of drugs and other chemicals into human milk. Pediatrics 2001;108:776-89.
423. Makris A, Thornton C, Hennessy A. Postpartum hypertension and nonsteroidal analgesia. Am.J.Obstet.Gynecol. 2004;190:577-8.
424. Greer IA. Epidemiology, risk factors and prophylaxis of venous thrombo-embolism in obstetrics and gynaecology. Baillieres Clin Obstet Gynaecol. 1997;11:403-30.
425. Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth.Pain Med. 2003;28:172-97.
426. Brown M. Renal complications in the normal pregnancy. In Johnson RJ, Feehally J, eds. Comprehensive clinical nephrology, Toronto: Mosby, 2003.
427. Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur.J.Obstet.Gynecol.Reprod.Biol. 2002;101:6-14.
428. Lin J,.August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet.Gynecol. 2005;105:182-92.
429. Dixon JB, Dixon ME, O’Brien PE. Pregnancy after Lap-Band surgery: management of the band to achieve healthy weight outcomes. Obes.Surg. 2001;11:59-65.
430. Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I–Blood pressure measurement, diagnosis and assessment of risk. Can.J Cardiol. 2006;22:573-81.
431. Khan NA, McAlister FA, Rabkin SW, Padwal R, Feldman RD, Campbell NR et al. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part II – Therapy. Can.J Cardiol. 2006;22:583-93.
432. Williams D. Pregnancy: a stress test for life. Curr.Opin.Obstet.Gynecol. 2003;15:465-71.
433. Vikse BE, Irgens LM, Bostad L, Iversen BM. Adverse perinatal outcome and later kidney biopsy in the mother. J Am.Soc.Nephrol. 2006;17:837-45.
434. Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am.J.Kidney Dis. 2003;42:982-9.
435. Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am.J.Epidemiol. 2004;159:336-42.
436. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002-6.
437. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 2003;326:845.
438. Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am.J.Kidney Dis. 2003;42:982-9.
439. van Walraven C, Mamdani M, Cohn A, Katib Y, Walker M, Rodger MA. Risk of subsequent thromboembolism for patients with pre-eclampsia. BMJ 2003;326:791-2.
440. Bar J, Kaplan B, Wittenberg C, Erman A, Boner G, Ben Rafael Z et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol.Dial.Transplant. 1999;14:1129-32.
441. North RA, Simmons D, Barnfather D, Upjohn M. What happens to women with preeclampsia? Microalbuminuria and hypertension following preeclampsia. Aust.N.Z.J Obstet Gynaecol. 1996;36:233-8.
442. Shammas AG,.Maayah JF. Hypertension and its relation to renal function 10 years after pregnancy complicated by pre-eclampsia and pregnancy induced hypertension. Saudi.Med.J 2000;21:190-2.
443. Genest J, Frohlich J, Fodor G, McPherson R. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. CMAJ. 2003;169:921-4.
Authors #
1-4Magee LA, MD, Clinical Associate Professor of Medicine;
5Helewa M, MD, FRCSC, Professor of Obstetrics and Gynaecology;
6,7Rey E, MD, FRCPC, Professor of Medicine and Obstetrics and Gynaecology;
8Côté AM, MD, FRCPC, Assistant Professor of Medicine;
9Douglas J, MD, FRCPC, Professor of Anaesthesia;
10Gibson P, MD, FRCPC, Assistant Professor of Medicine;
11Gruslin A, MD, FRCSC, Associate Professor of Obstetrics and Gynaecology;
12Lange I, MD, FRCSC, Professor of Obstetrics and Gynaecology;
7Leduc L, MD, FRCSC, Professor of Obstetrics and Gynaecology;
13Logan AG, MD, FRCPC, Professor of Medicine;
14Smith GN, Professor of Obstetrics and Gynaecology;
1,2,14-16STIRRHS Pre-eclampsia Scholars (Appendix 1);
1Cardew S, MD, Resident in Internal Medicine;
2Firoz T, MD, Resident in Internal Medicine;
17Moutquin J-M, MD, FRCSC, Professor of Obstetrics and Gynaecology;
2-4von Dadelszen P, MBChB, DPhil, FRCSC, FRCOG, Associate Professor of Obstetrics and Gynaecology.
1Department of Medicine, University of British Columbia, Vancouver, BC;
2Department of Obstetrics and Gynaecology, University of British Columbia, Vancouver, BC;
3Centre for Applied Health Research and Evlauation, Child and Family Research Institute, University of British Columbia, Vancouver, BC;
4Department of Healthcare and Epidemiology, University of British Columbia, Vancouver, BC
5Department of Obstetrics and Gynaecology, University of Manitoba, Winnipeg, MB;
6Departments of Medicine, University of Montréal, Montréal, QC;
7Department of Obstetrics and Gynaecology, University of Montréal, Montréal, QC;
8Department of Medicine, University of Sherbrooke, Sherbrooke, QC;
9Department of Anaesthesiology, Pharmacology and Therapeutics, University of British Columbia, Vancouver, BC;
10Department of Medicine, University of Calgary, Calgary, AB;
11Department of Obstetrics and Gynaecology, University of Ottawa, Ottawa, ON;
12Department of Obstetrics and Gynaecology, University of Calgary, Calgary, AB;
13Department of Medicine, University of Toronto, Toronto, ON;
14Department of Obstetrics and Gynaecology, Queens University, Kingston, ON;
15Department of Obstetrics and Gynaecology, McGill University, Montréal, QC;
16Department of Obstetrics and Gynaecology, University of Alberta, Edmonton, AB;
17Department of Obstetrics and Gynaecology, University of Sherbrooke, Sherbrooke, QC.
