Description #
Review current state of pain management
Physiological differences between Acute, Neuropathic and Chronic pain
Learning Objectives #
At the end of this course the health care professional will be able to:
- a. Define and classify pain
- b. Epidemiology and Impact of Pain
- c. Describe key mechanisms of pain physiology of Acute, Neuropathic & Chronic Pain
- d. Case study introduction
Pain #
Definition of Pain:
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or describe in terms of such damage. (IASP, APS)
Pain is whatever the experiencing person says it is, existing whenever he says it does (McCaffery, 1986). Although pain is a universal experience, it is an individual subjective experience subjective and the person not the healthcare professional is the authority on his/her pain.
Why do we need to talk about Pain?
- There is a major gap between understanding of physiology, pathophysiology of pain and the assessment, management and treatment of pain.
- One of the most common issues dealt with by healthcare providers on a daily basis.
- Pain education for healthcare providers is generally inadequate. Improvement in pain management practices are significantly impacted by the lack of adequate education, assessment skills, attitudes towards pain as well as a lack of resources for patients (Chronic Pain resources such as Chronic pain clinicians and interdisciplinary teams and acute pain services). Bringing research into practices in pain management continues to be a challenge and remains suboptimal in many acute and chronic pain patients (Jovey 2008. Macintyre & Schug 2007).
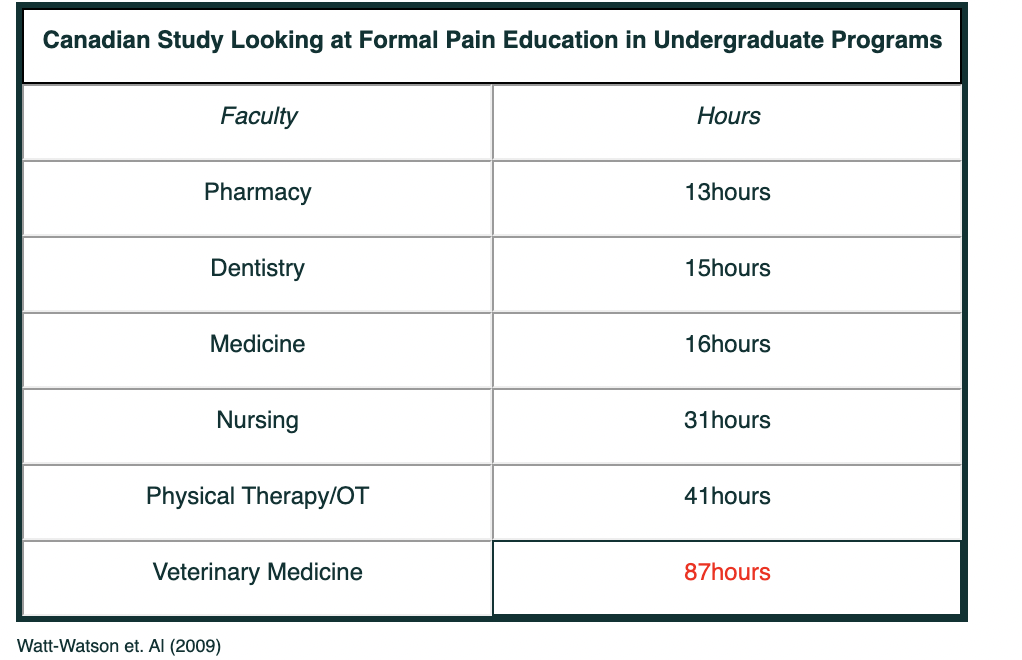
Based on a study by Judy Watt-Watson et al. showed that only 1/3 of the University Programs could identify and formal pain education.
The following table shows the allotted time used for Pain education in different faculties.
Canadian Study Looking at Formal Pain Education in Undergraduate Programs
What do we know about Pain so far?
- Pain is a subjective, personal experience.
- 41-80% of patients report moderate to severe pain in the postoperative period (Sommer, 2008. Apfelbaum, 2003).
- More than 70% of cancer patients have pain & 30% die in uncontrolled pain (Goldman, 2003).
- 20-25% of the adult population suffers from chronic pain (Jovey, 2007).
Classification of Pain:
Pain is classified in various ways:
- Pain duration – Acute or Chronic
- Physiologically – Nociceptive or neuropathic
Acute Pain
Acute Pain is recent onset pain, which can last from minutes to day/week (less than 1-3 months). It is associated with tissue damage and some degree of inflammation.
It is usually somatic (bone, joint, muscle, skin or connective tissue) and or visceral (organs). Acute pain is often associated with a degree of inflammation. It is a protective mechanism and a warning of injury or disease. It is easily diagnosed and has an obvious pathopysiology. If under treated, acute pain may go on to become chronic pain.
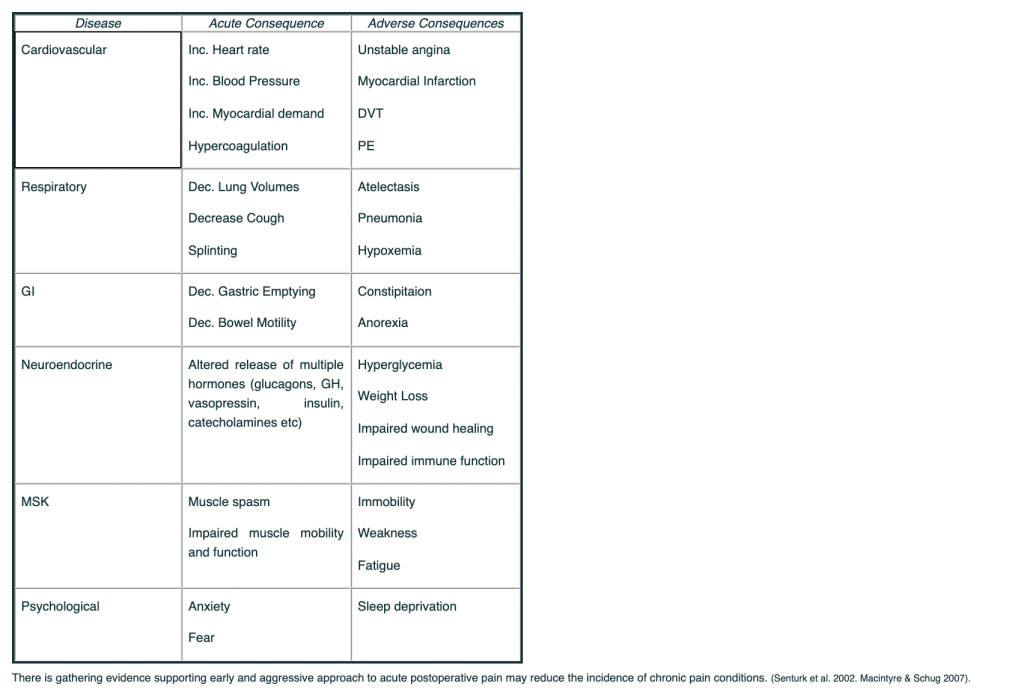
Adverse effect of unrelieved Pain:
This table summarizes some of the adverse physiological consequences of unrelieved acute pain – in the surgical or post injury patient. Besides the adverse consequences outlined in the table, under-treated acute pain may increase the risk of chronic pain.
Chronic Pain
Chronic Pain is a persistent pain beyond the usual course of recovery time for injury or illness (greater than 3 months), or intermittently reoccurs over months or years. Chronic pain affects the quality of life as well as function with often-significant impact to the emotional and physical well being of the person.
Any surgical or injury can go onto become chronic pain.
This table highlights some of the more common surgeries where pain becomes chronic:

Chronic Pain is now known to be pathological changes in the peripheral and central nervous systems. It persists beyond the usual course of injury, not physically protective.
An alteration of the “normal” pain transmission mechanism which is a consequence of maladaptive changes that includes peripheral sensitization, central sensitization and reduced inhibitory (modulation) activity.
The impact of chronic pain can often include sleep dysfunction, fatigue, mood (depression), decreased quality of life and ADL’s.
Peripheral sensitization: Inflammatory mediators as well as intense repeated or prolonged noxious stimulation can sensitize nociceptors – these causes a lower threshold for activation and therefore an increase rate of firing and increased resistance to modulation.
In turn increased activity along the nociceptors causes changes within the dorsal horn of the spinal cord called central sensitization. It activates additional receptors such as the NMDA receptor via excessive glutamate dislodging the magnesium ion that normally stops the NMDA receptor from being activated. It is thought that the activation of the NMDA receptor in one of the first steps in central hyper-sensitisation and hyper-responsiveness to nociception and aberrant neural input. As well the modulation or anti-nociceptive system producing endorphins and other naturally intrinsic pain relievers cannot keep up with the increased transmission activity along the spinal neurons transmitting the pain.
Changes that may occur in Chronic Pain:
1. Windup:
- Turns up the “volume” and intensity of signal with less stimulation needed.
- Increased firing of pain signals (changes in the Na+ channel).
- Recruitment of other sensor fibers that do no usual transmits pain (C and Aβ fibers).
- [?]Hyperalgesia[/?] (Hypersenstivity).
- Allodynia (touch interrupted as pain – diabetic neuropathies, chronic degenerative arthiritis).
- Increased perception of pain (increased input to “pain sensing areas” of the brain.
- Opioid resistance (peripheral, spinal cord and brain).
- Changes in the ion channels (Na+, Ca+, K+ and chemical mediators nerve growth factor, glutamate, Substance P and NO).
2. Long-Term:
- Nerve fiber plasticity – “rewiring” of pain transmission in the peripheral and CNS sprouting of new nerve fibers and interconnections.
- Cell death in spinal nerve cells.
- Novel transmission (reverse transmission, increase release inflammatory chemicals and ectopic neuronal pacemaker sites): It includes spontaneous firing without stimulus, ectopic sites – where the impulse will start in areas other than the initial nerve fiber cells.
- Pain memory (embedded in the spinal cord): Example: Pain memory within the nerve fibers and spinal cord is phantom limb pain. Even after the limb is removed up to 80% of patients will have some sensation or pain in the missing limb.
Neuropathic Pain
Neuropathic pain is caused by an injury or damage to the peripheral or central nervous system (Jovey, 2002). It affects about 2-3% of the general adult population. An infection, trauma, ischemia, metabolic disorders, tumors, toxins or primary neurological diseases usually causes neuropathic pain. It is often severe and disabling in nature and difficult to manage.
Examples of peripheral neuropathic pain include – Painful diabetic neuropathy (20-24%), Postherpetic neuralgia (25-50%), Phantom Limb Pain (30-85%),
Central Post Stroke pain (2-8%).
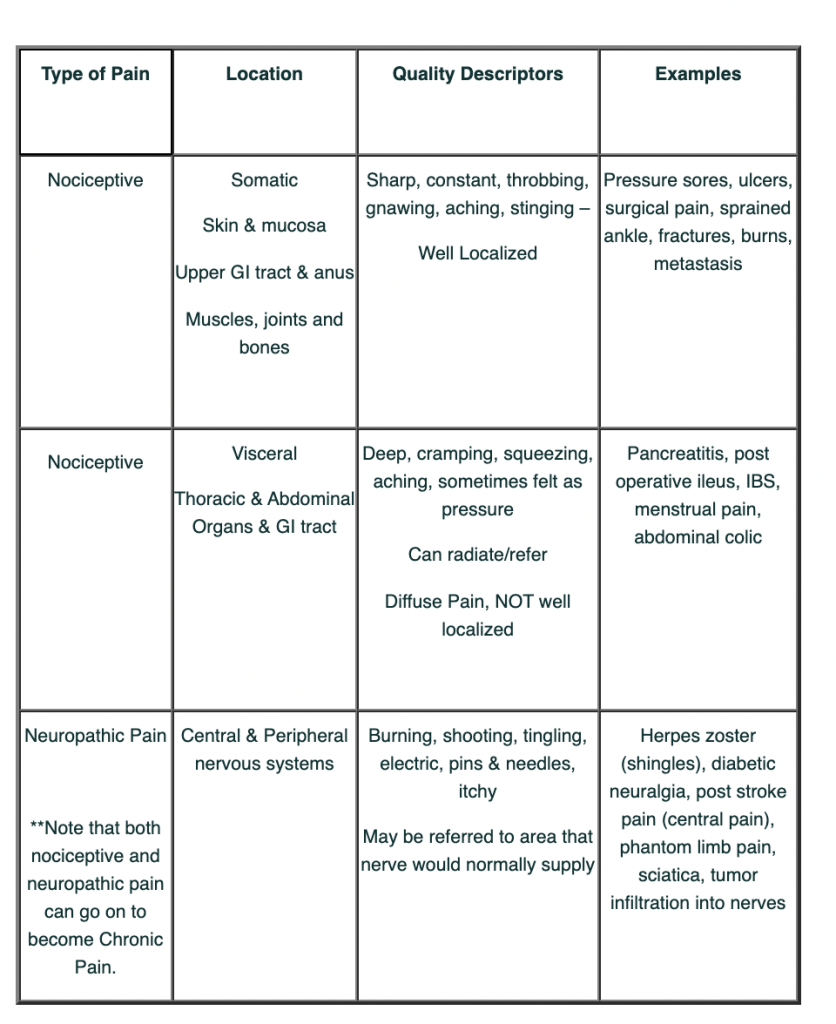
Examples
This table has some examples of nociceptive and neuropathic pain.
Pain Physiology
Nociception is the process by which tissue damage information is conveyed from the site of injury to the brain and perceived as pain. It is a series of complex electro-chemical events that occur between the site of tissue inflammation/damage and the perception of pain. The physiology of pain perception has been broken down into 6 categories: Transduction, Transmission, Modulation, Perception, Interpretation and Behavior.
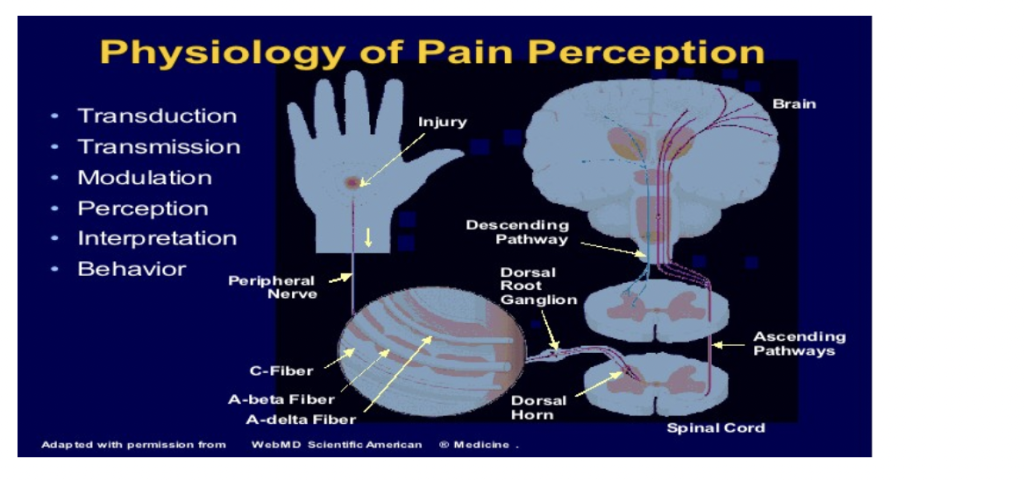
Transduction: Nociceptors respond to noxious stimuli (mechanical, thermal and chemical). The nociceptors are activated and release molecules and mediators of inflammation (prostaglandins, bradykinin, serotonin, substance P, cytokines and histamine).
Transmission: Three types of nociceptors transmit via noxious stimulus via electrical activity (via activation of voltage gated Na+ channels) from the periphery to the dorsal horn of the spinal cord.
The three types are:
- Aβ fibers are large, myelinated and very fast (V40-50m/sec). They carry sensation associated with fine touch, pressure and vibration. These fibers are not usually involved in pain transmission but do play a role as acute pain becomes persistent or chronic in nature.
- Aδ fibers are small, myelinated and fast (>10 and <40-50m/sec). They carry sensation associated with fine touch, pressure and vibrations. These fibers are not usually involved in pain transmission but do play a role as acute pain becomes persistent or chronic in nature.
- C fibers are small, slow (<2m/sec) and unmyelinated. They carry sensation associated with temperature (heat), slow and dull pain. They respond in the same fashion to all noxious stimuli – termed polymodal nociceptors. A number of these C fibers are “silent” or non-active unless there is high and continuous stimulation. These “silent” C fibers play a role in sensitized and are involved in persistent or chronic pain when the volume is “turned” up from spontaneous firing along the nerve fibers.
Neurotransmitter: The main neurotransmitter from the nociceptors and synapse –> dorsal horn neurons in the spinal cord is Glutamate.
Perception:
Pain becomes a conscious experience in a number of areas of the brain:
- Thalamus is the main relay center for sensory inputs.
- Reticular system is believed to be responsible for autonomic response to pain (Give warning signs).
- Somatosensory cortex interprets, localizes and characterizes pain.
- Limbic System is responsible for the emotional and behavioural aspects to pain are perceived.

Anti-Nociceptive System
The Pain-Relieving Anti-nociceptive System
The body’s pain relieving, or anti-nociceptive, system balances out the pain-sensing system. When pain signals transmitted by peripheral nerves, or nociceptors, arrive in the brain, they activate neurons in the periaqueductal gray matter of the brain and the nucleus raphe magnus of the brainstem, which release endorphins and enkephalins.
Endorphins bind to opioid receptors within the periphery and dorsal horn, inhibiting the transmission of more pain signals. Enkephalins bind to delta-opioid receptors in the spinal cord, causing spinal neurons to release gamma-aminobutyric acid (GABA), alpha-2 adrenergic mediators, oxytocin, relaxin, and other chemicals that inhibit the pain signals in the spinal cord. Many medications used for pain relief mimic the actions of these natural analgesic agents.
In addition to pain signals, other stimuli can trigger activation of the anti-nociceptive system, such as exercise, meditation, and being comforted or reassured. This explains the utility of many of the behavioral components of pain management programs.
This system is shown the diagram below:

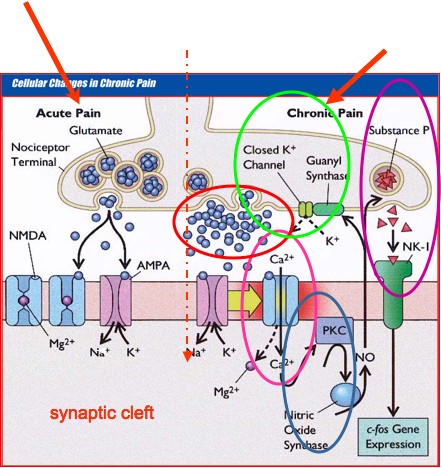
Pathophysiology of Pain
Pathophysiology of Pain:
- Chronic Pain induces multiple changes at the cellular level.
- Opening of the NMDA receptor via Ca2+ channel due to prolong activation of the AMPA receptor (Windup, neural remodeling).
- Protein kinase (PKC) is activated which is used to produce nitric oxide (NO).
- NO stimulates guanyl synthase to close K+ channels. This causes opiate resistance as endorphins, enkephalins and opioids inhibit pain by opening K+ channels.
- NO stimulates substance P and triggers c-fos gene expression, which promotes neural remodeling and hypersensitization.
The following diagram shows the pathophysiology of pain:
Pain Management
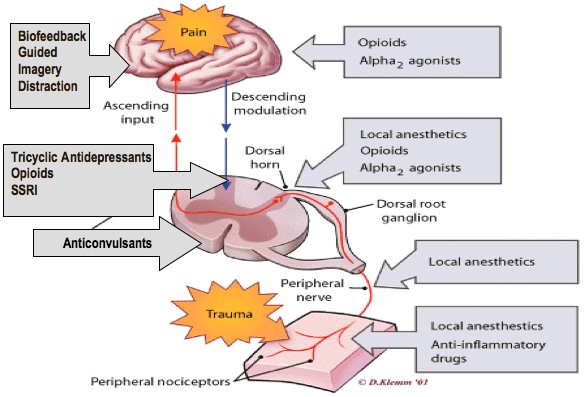
The diagram shown above explains the pain management options at different stages of Pain transmission/reception.
Nociceptive Pain Management:
The management strategies for acute pain are very different than chronic pain. The emphases is on pharmacological – nonopioids, nsaids & opioids depending on the type of injury/surgery with less of a focus on the psychological. The expectation is that over a period of time most acute pain will resolve.
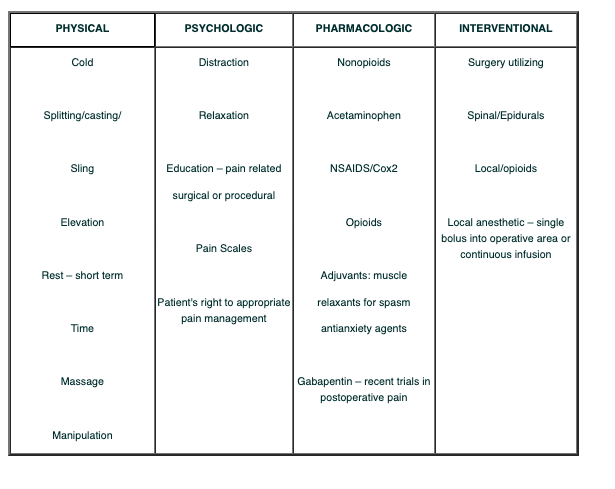
The table below shows the physical, psychological, pharmacological and interventional aspects of Pain management.
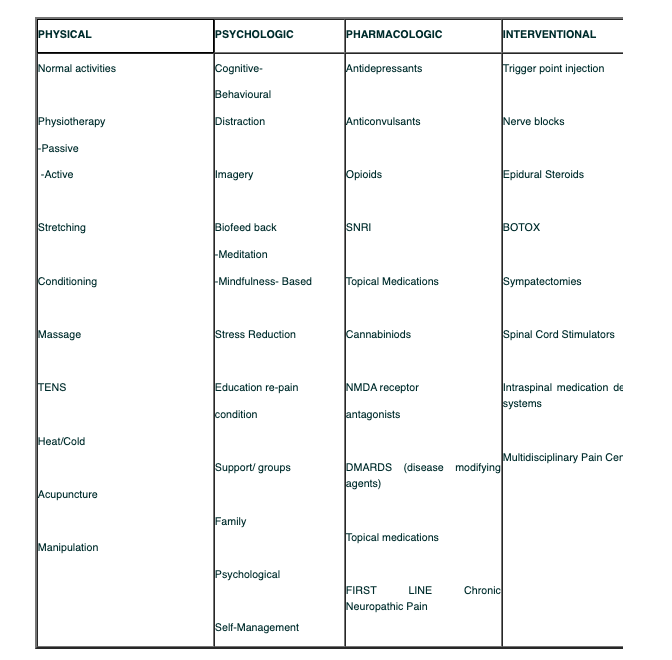
Treatment Options for Neuropathic/Chronic Pain:
This table shows the treatment options of Neuropathic and Chronic pain:
Lecture Presentation***
References:
- Apfelbaum, J., Chen, C., Mehta, S., Tong, J. (2003). Postoperative Pain Experience: Results from a National Survey Suggest Postoperative Pain Continues to Be Undermanaged. Anesthetic Analog, 97: 534-40.
- Brookoff D. Chronic Pain: A New Disease? Hosp Pract (Off Ed) 2000;35:45-52, 59.
- Goldman, R. (2003). Strategies for Cancer Pain. http://www.paincare.ca/site/download/en/featured_articles/sharing_knowledge_cancer.pdf
- Goldman, B (2008). Managing Pain: The Canadian Healthcare Professional’s Reference. Baker Edwards Consulting Co. Ont.
- Jovey, R. (2007) Pain Focus. http://chronicpainmanagement.ca/sitebuildercontent/sitebuilderfiles/painfocusvol1.pdf
- Macintyre, P., Schug, S. (2007). Acute Pain Management, A Practical Guide. Sauders Elsevier, Toronto.
- McCaffery, M., Pasero, C. (1999). Pain: clinical manual. Mosby, Inc. St. Louis.
- Moulin, DE, Clark AJ, Speechley M, Morley-Forster PK. Chronic pain in Canada- prevalence, treatment, impact and the role of opioid analgesia. Pain Res. Manag. 2002: Tables and Figures: Manuscript Version.
- National Pharmaceutial Council & Joint Commission on Accreditation of Healthcare Organizations (2001). Pain: Current Understanding of Assessment, Management & Treatments. www.npcnow.org/resources/PDFs/painmonograph.pdf assessed July,2010.
- Sommer, M. et al. (2008). The prevalence of postoperative pain in a sample of 1490 surgical inpatients. European Journal of Anaesthesiology., 25:267-274 Cambridge University Press.
- Statistics Canada http://www.statcan.gc.ca/daily-quotidien/080221/dq080221b-eng.htm assessed July, 2010.
